HIGHLIGHTS
•
A copper-rhodium photocatalyst has been found to convert methane and water to hydrogen in an emission-free steam methane reforming process conducted in the presence of light.
•
Both copper and rhodium are required to produce the reaction’s other product, carbon monoxide, selectively.
•
The photocatalyst exhibits a space-time yield of 15.6 millimoles per centimeter per second which is approximately 15 times higher than what is observed for large catalytic processes.
Syngas (hydrogen and carbon monoxide) is an important raw material used in the manufacture of hydrocarbon liquids and waxes. When syngas is heated to a temperature between 200°C and 350°C in the presence of cobalt or iron catalysts, one of the key products, from this process known as the Fischer-Tropsch reaction, is a gas-to-liquid (GTL) lubricant base stock.
The Fischer-Tropsch reaction has been used for about 100 years, but there has been no consensus on its mechanism. In a past TLT article,
1 researchers determined that the Fischer-Tropsch reaction can vary over specific time frames and temperature due to an oscillation effect. Changing the ratio of the two reactants can impact the oscillation behavior. As the reaction temperature increases, the reaction slows down because the two reactants (which are gases) lose contact with the catalyst surface. Once the reaction slows down, the temperature declines allowing the starting materials to enrich the catalyst surface, increasing reaction output.
The primary pathway for producing hydrogen and carbon monoxide is by reacting methane with water in the steam methane reforming process. Peter Nordlander, Wiess chair and professor of physics and astronomy and professor of electrical and computer engineering, and materials science and nanoengineering at Rice University in Houston, Texas, says, “The steam methane reforming reaction is highly energy intensive as it is conducted under high temperature and pressure conditions. The process uses coking (heating coal or petroleum to a high temperature) and involves a high degree of carbon intensity. As decarbonization moves forward, the methane reforming process is the leading pathway for producing hydrogen.”
Ironically, the grey hydrogen generated in this manner for use in sustainable processes is synthesized in an unsustainable manner. Hydrogen can be produced in a more sustainable manner such as through electrolysis of water. The challenge is that a cost-effective, low energy procedure has not been identified.
Naomi Halas, Stanley C. Moore professor of electrical and computer engineering at Rice University, offers an alternative strategy known as photocatalytic steam methane reforming. She says, “Substitution of light instead of heat can reduce the activation energy of steam methane reforming leading to a process that can be run at a lower temperature and is more energy efficient.”
Attempts to use photocatalysis have been limited to ultraviolet illumination or required additional external heating. But plasmonic photocatalysis has been shown to be effective in specific applications.
Halas says, “Light excitation of specific metals energizes electrons leads to their interaction with positive ions generating plasmons. These species are very efficient light absorbers that can produce metal nanoparticles that are highly reactive with specific substrates. This light induced reaction process has been found to be effective with various metal systems including gold, silver, copper and aluminum.”
Halas, Nordlander and their colleagues have now found the right catalyst combination to utilize plasmonic photocatalysis to form hydrogen in a more efficient manner than using conventional steam methane reforming.
Copper-rhodium antenna-reactor photocatalyst
The researchers found that when exposing a copper-rhodium (Cu-Rh) photocatalyst to white laser illumination in the presence of 3% methane and 3% water (the remainder is helium) a high yield of hydrogen was produced
(see Figure 3). In conducting the photocatalytic reaction, the two reactants were fed into a fixed bed continuous flow reactor at ambient temperature. Nordlander says, “In the present laboratory scale experiment which used very diluted concentrations of methane and water, we were able to achieve an external quantum efficiency of 2%, which can be further improved by increasing the partial pressure of methane and water, and by using a more optimized reactor.”
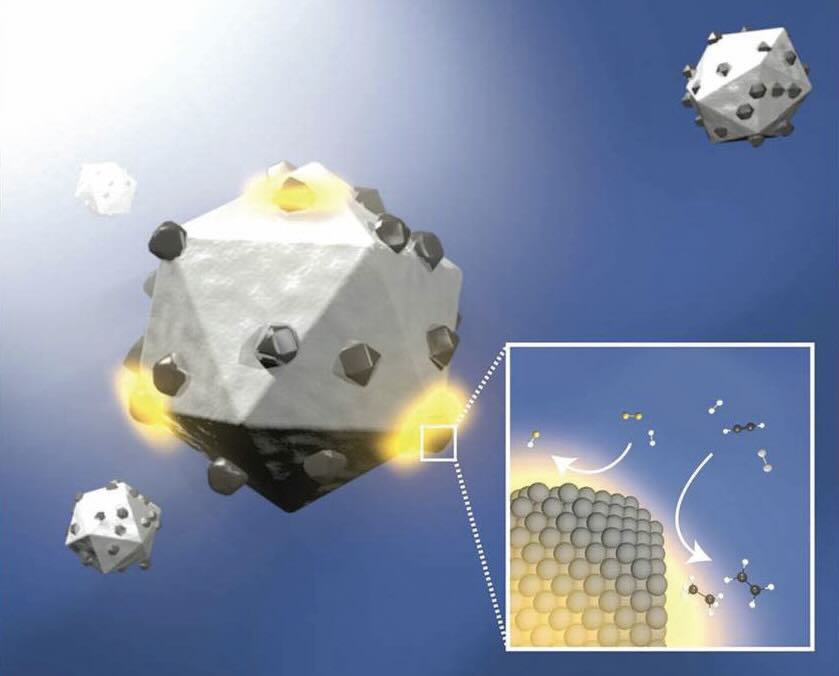 Figure 3. Copper is used as a substrate for rhodium (small particles on the catalyst surface), which enables this antenna photocatalyst to convert methane and water to hydrogen. An image in the right-hand corner of the figure displays the conversion to hydrogen. Figure courtesy of Rice University.
Figure 3. Copper is used as a substrate for rhodium (small particles on the catalyst surface), which enables this antenna photocatalyst to convert methane and water to hydrogen. An image in the right-hand corner of the figure displays the conversion to hydrogen. Figure courtesy of Rice University.
Catalyst selection was based on finding a metal that will generate plasmons upon exposure to light. Nordlander says, “A good deal of attention has been paid to using noble metals such as platinum and rhodium that have a large quantum efficiency.” Halas continues, “Copper is not reactive toward light and is widely available making it a good material to use as the substrate (or antenna) for another metal. We selected rhodium as the second metal and prepared various ratios of this metal with copper to optimize performance. Nanoparticles of the copper-rhodium antenna photocatalyst with an average size of 15 nanometers were evaluated. We achieved superior performance, stability and selectivity with a photocatalyst exhibiting the following stoichiometry: Cu
19.5Rh
0.5.”
The researchers noted that using copper or rhodium by themselves led to a lower degree of product selectivity. Both metals are required to catalyze the reaction and produce carbon monoxide selectively. Nordlander says, “Methane’s carbon atoms bind to rhodium and not to copper because of greater binding affinity. The rhodium specks in the copper are able to bind water and methane molecules to the plasmonic surface producing conditions for the reaction to move to completion. At the completion of the reaction, carbon monoxide desorption occurs selectively.”
Conducting thermocatalytic steam methane reforming at temperatures ranging from 250 to 550°C, using the copper-rhodium antenna photocatalyst, was not as selective and suffered from unstable reactivity at a fast decay rate. Halas says, “We determined that exposing the depleted catalyst to the white light illumination led to complete regeneration.”
The turnover frequency, a measure of the photocatalyst performance, for hydrogen production was determined to be 2.99 micromoles of hydrogen per milligram of rhodium per second. After normalization, this value translates into a space-time yield of 15.6 millimoles per centimeter per second, which is approximately a 15 times higher rate than what is observed for large catalytic processes.
Halas says, “This finding demonstrates the potential for using the copper-rhodium photocatalyst to produce grey hydrogen in a sustainable manner.”
Future work will involve scaling up the photocatalytic process toward commercialization. Nordlander adds, “We will also be trying to improve the photocatalyst by replacing the copper antenna with aluminum and also identifying a more sustainable option to replace rhodium.”
Additional information can be found in a recent article
2 or by contacting Halas at
halas@rice.edu and Nordlander at
nordland@rice.edu.
REFERENCES
1.
Canter, N. (2024), “New insight into the Fischer-Tropsch reaction,” TLT,
80 (2), pp. 14-15. Available at
www.stle.org/files/TLTArchives/2024/02_February/Tech_Beat_II.aspx.
2.
Yuan, Y., Zhou, J., Bayles, A., Robatjazi, H., Nordlander, P. and Halas, N. (2024), “Steam methane reforming using a regenerable antenna-reactor plasmonic photocatalyst,”
Nature Catalysis, 7, pp. 1339-1349.