Sustainable, sulfurized, inorganic-organic hybrid networks were developed as cathodes in lithium-sulfur batteries.
The use of an organic polymer such as polyacrylonitrile produced a conjugated sp2 framework that facilitates electron transport.
Testing of the hybrid polymer network cathode in a full battery cell led to a higher specific capacity and improved capacity retention than a conventional lithium-sulfur battery.
The general trend in the development of batteries for use in applications such as electric vehicles has focused on maximizing the performance, reliability and safety of lithium-ion batteries. In parallel, research is continuing on the development of high-performance lithium batteries that are based on cheaper and more readily available raw materials with continuing concerns about sourcing such metals as cobalt and nickel for lithium-ion battery cathodes.
One example is the growing interest in lithium-metal batteries where the anode consists of lithium metal and the cathode is based on sulfur. A previous TLT article
1 discussed research conducted to better understand if the accepted failure mechanism for lithium-metal batteries involves the generation of dendrites at the lithium-metal anode that penetrate the polymer separator and eventually reach the cathode causing a short-circuit. To better understand how lithium-metal batteries fail, researchers utilized a femtosecond laser to mill through a battery’s outer casing after 101 cycles and used a scanning electron microscope to study the inner workings of the battery. Dendrites were not found to be the cause of failure but rather a pathway created by the solid electrolyte interphase enabled lithium metal to penetrate and tear the plastic separator. In addition, large deposits of lithium-metal were also found.
Lithium-sulfur batteries have potential to be used due to a high energy density. The low cost and wide availability of sulfur are also attractive features. But development of lithium-sulfur batteries has been hindered by inferior performance.
Donghai Wang, Brown Foundation chair of mechanical engineering and professor of mechanical engineering at Southern Methodist University in Dallas, Texas, says, “Lithium-sulfur batteries exhibit poor cycle stability due to sluggish kinetics and drastic volume changes. The problem can be traced to the formation of polysulfide intermediates during the cycling process and their dissolution in the liquid electrolyte, which is based typically on a carbonate solvent. The polysulfide species are formed when lithium ions being cycled react with sulfur atoms at the cathode. The dissolution process leads to degradation of the cathode.”
Attempts to overcome this performance limitation included the preparation of organic polymers as sulfur cathodes by covalently bonding small sulfur species onto conductive carbon backbones. While this approach has the benefit of cost-effectiveness and sustainability, the conversion between small molecules and lithium sulfide produces limited battery capacities due to the short length of the bonded sulfur chains and sparse carbon-sulfur bonding sites in the organic polymers.
A new approach has now been developed to minimize solubilization of polysulfide intermediates, which leads to the creation of a lithium-sulfur battery with high capacity and excellent cycling stability.
Hybrid polymer network cathode
Wang and his colleagues constructed sustainable, sulfurized inorganic-organic hybrid polymer networks as the sulfur cathodes. The hybrid polymer was formed through hybridization of an inorganic sulfurized polyphosphazene that contained a high degree of sulfur content.
Wang says, “Our strategy was to take advantage of the alternating phosphorus and nitrogen sites on the phosphazene. Phosphorus atoms are available to graft sulfur chains and nitrogen atoms are positioned for onsite absorption of lithiated sulfur species. Use of an organic polymer such as polyacrylonitrile enables the formation of a conjugated sp
2 framework that will facilitate electron transport essential for strong battery performance.”
The concept is demonstrated in Figure 1 where sulfur chains are immobilized by covalent tethers and lithium sulfide discharge products are regulated by onsite atomic adsorption and hybrid polymeric frameworks. Figure 2 illustrates a more detailed view of the proposed process.
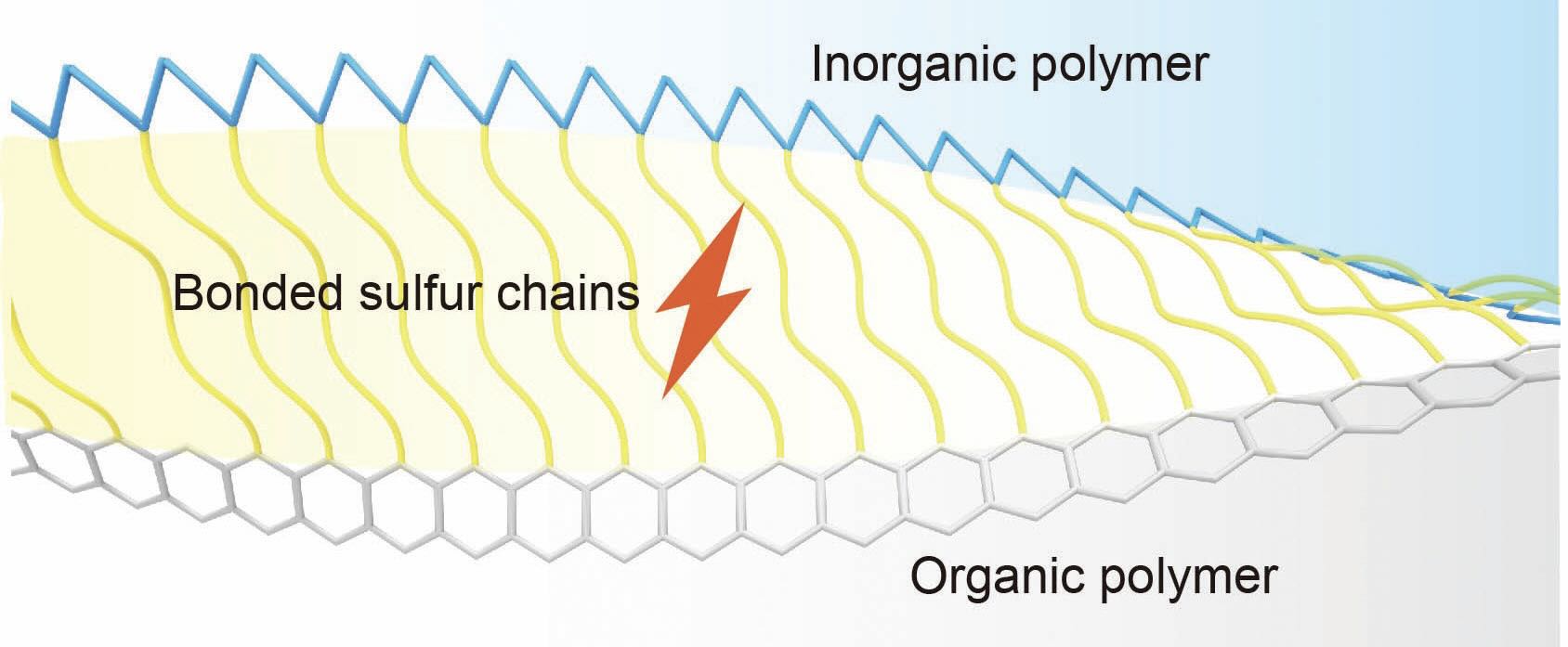 Figure 1. A schematic of the sulfurized polymer hybrid network illustrates the proposed way that sulfur chains are tethered to organic and inorganic polymers. Figure courtesy of Southern Methodist University.
Figure 1. A schematic of the sulfurized polymer hybrid network illustrates the proposed way that sulfur chains are tethered to organic and inorganic polymers. Figure courtesy of Southern Methodist University.
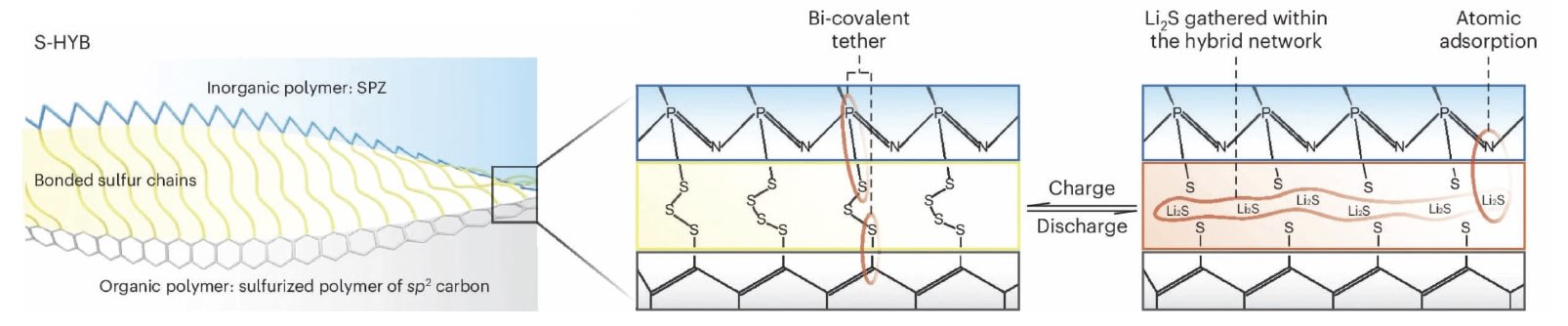
Figure 2. A more detailed view of the composition of the sulfurized polymer hybrid network demonstrates how the bonding among atoms may change during a charge, discharge cycle. Figure courtesy of Southern Methodist University.
Elemental analysis of the hybrid polymer confirmed that the sulfur content was higher (approximately 62%) than a conventional lithium-sulfur cathode (approximately 51%). An
in situ transmission electron microscope technique was used to study in real-time the lithiation and delithiation of the hybrid polymer network cathode. The result was that the hybrid polymer network initiated a unique inserting conversion reaction that differs from past approaches. Nano-crystalline lithium sulfide was formed from the amorphous network and then converted back to an amorphous state. No volume expansion nor microstructural collapse was observed.
The next step was evaluation of the hybrid polymer network cathode in a full battery cell containing a carbonate-based liquid electrolyte. Wang says, “We found that the lithium-sulfur battery based on the hybrid polymer network cathode exhibited higher specific capacity (over 900 milliamps per gram) and improved capacity retention (nearly 88% after 300 cycles) than a conventional lithium-sulfur battery. No evidence was found for soluble polysulfide formation when using the hybrid polymer network cathode. This finding was supported by the high sulfur utilization (87%), which is a demonstration of the ability of the cathode to unlock the high capacity of the high sulfur content.”
Similar results were seen when the hybrid polymer network cathode was included in lithium-sulfur pouch cell batteries. A capacity retention of nearly 85% was realized after 150 cycles with a projected energy density approaching 300 watt-hours per kilogram. Both figures are superior to conventional lithium-sulfur batteries under similar operating conditions.
The sustainability aspect of this lithium-sulfur battery was due to the utilization of pitch from waste paving materials and nitrile rubber from scrap tires. Wang says, “We were able to process both recycled materials into a hybrid polymer network cathode with comparable performance to what was observed using virgin starting materials.”
This approach furnishes an opportunity to develop high-performance lithium-sulfur batteries and opens a strategy for doing it in a sustainable manner. Wang indicates the next step in the project is to scale up the technology to produce a large battery cell while gaining a better understanding of the mechanism for how the hybrid polymer network cathode is so effective.
Additional information can be found in a recent article
2 or by contacting Wang at
donghaiwang@smu.edu.
REFERENCES
1.
Canter, N. (2021), “Lithium-metal batteries: Direct evidence for failure,” TLT,
77 (10), pp. 12-13. Available at
www.stle.org/files/TLTArchives/2021/10_October/Tech_Beat_I.aspx.
2.
Liao, M. Xu, Y., Rahman, M. Tan, S., Wang, D., Wang, K. Dandu, N., Lu, Q., Li, G., Le, L., Kou, R., Jiang, H., Nguyen, A., Shi, P., Ye, L., Ngo, A., Hu, E., Wang, C. and Wang, D. (2024), “Hybrid polymer network cathode-enabled soluble-polysulfide-free lithium-sulfur batteries,”
Nature Sustainability, 7, pp. 1709-1718.