HIGHLIGHTS
•
A process known as hydrodynamic cavitation shows potential for removing PFAS from wastewater by releasing an intense amount of energy through the generation and collapse of bubbles.
•
During the collapse of the bubbles, powerful shock waves are produced that can cause spikes in pressure and temperature leading to degradation of PFAS up to a rate of 55% over a short period of time.
•
Pilot testing of this technology was successfully undertaken at a Stockholm, Sweden, municipal wastewater plant.
The presence of per- and polyfluoroalkyl substances (PFAS) in the environment has become a significant concern for health and safety reasons. PFAS are highly resistant to degradation under a wide range of conditions due to the exceptional stability of the carbon-fluorine bond. Developing strategies for degrading PFAS is a significant challenge.
In a past TLT article,
1 researchers determined that specific PFAS types can be degraded when exposed to a bacterium known as
Acetobacterium bakii. Enzymes within this bacterium are able to displace a fluorine atom attached to an unsaturated PFAS known as PFMeUPA within three weeks. But the bacterium does not remove fluorine atoms attached to saturated carbon atoms. While this approach is limited to only a few PFAS, the hope is that learning more about the mechanism will enable the design of enzymes that can decompose a wider range of PFAS.
Of particular concern is the presence of PFAS in wastewater that is generated at manufacturing facilities, industrial factories and leached from landfills. Dr. Morteza Ghorbani, assistant professor on microfluidics and cavitation at Sabanci University in Istanbul, Türkiye, says, “There is no current method for degrading PFAS on an industrial scale. Advanced reduction and oxidation processes such as ozonolysis, persulfate, hydrogen peroxide and ultraviolet radiation show promise in degrading PFAS. But these techniques have limitations due to cost, high energy requirements, the need to use hazardous chemicals and the formation of byproducts that are costly to remove.”
Dr. Iakovos Tzanakis, professor in engineering materials in the School of Engineering, Computing, and Mathematics at Oxford Brooks University in Oxford, UK, says, “One of the most common approaches for removing contaminants from effluent is reverse osmosis. This filtration approach will collect residual byproducts but then the issue is how to eliminate these materials.”
Ghorbani and Tzanakis turned to a new approach that now shows promise in degrading PFAS in wastewater.
Hydrodynamic cavitation
The researchers found that a process known as hydrodynamic cavitation displays the potential to remove PFAS from wastewater. Hydrodynamic cavitation involves producing bubbles by subjecting the wastewater to a reduction in pressure when passed through a restrictive area, such as short channels, within a hydrodynamic reactor. As the bubbles grow and then collapse, a significant amount of energy is released in the form of mechanical, thermal and chemical effects. This intense release of energy acts to degrade several PFAS compounds simultaneously by 36% on average over only a 30-minute period.
Tzanakis says, “The appeal of green hydrodynamic cavitation is that it is effective in degrading PFAS without the need for chemicals, and it is both cost and energy efficient as it does not rely on electricity. Our vision is for poor countries to use this modular technology in a portable set-up. Ghorbani adds, “The hydrodynamic cavitation reactor has the potential to be scaled up for commercial use and can be used in different effluent treatment applications where bacteria are involved.”
Pilot testing of this technology was conducted after the membrane bioreactor at a waste treatment plant in Stockholm, Sweden, using municipal wastewater. The configuration of the hydrodynamic cavitation microfluidic device, the heart of the bioreactor, is shown in Figure 3, and an image of the reactor is provided in Figure 4.
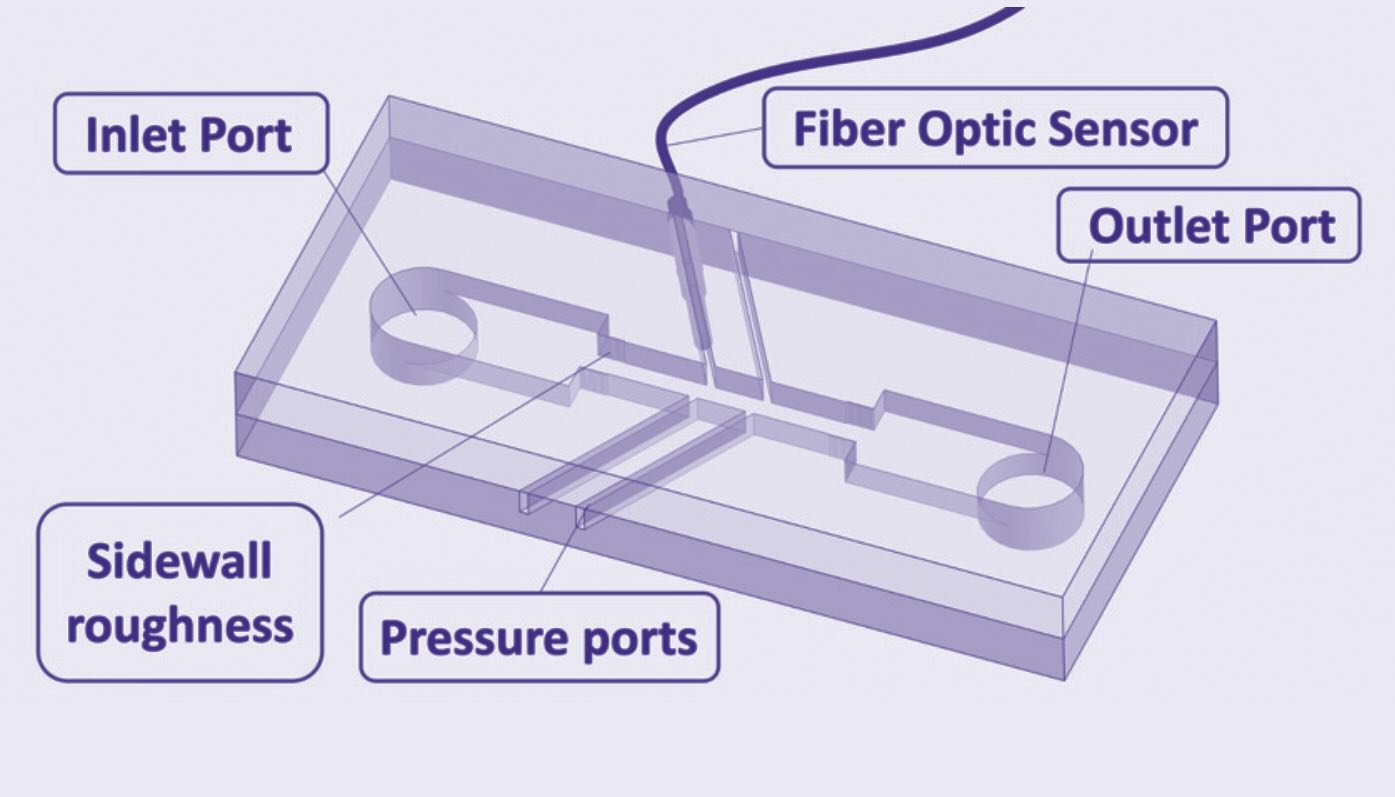
Figure 3. The design for a promising technology for removing PFAS from wastewater has been evaluated on a pilot scale and demonstrates potential to reduce levels in a short period of time. Figure courtesy of Oxford Brooks University.
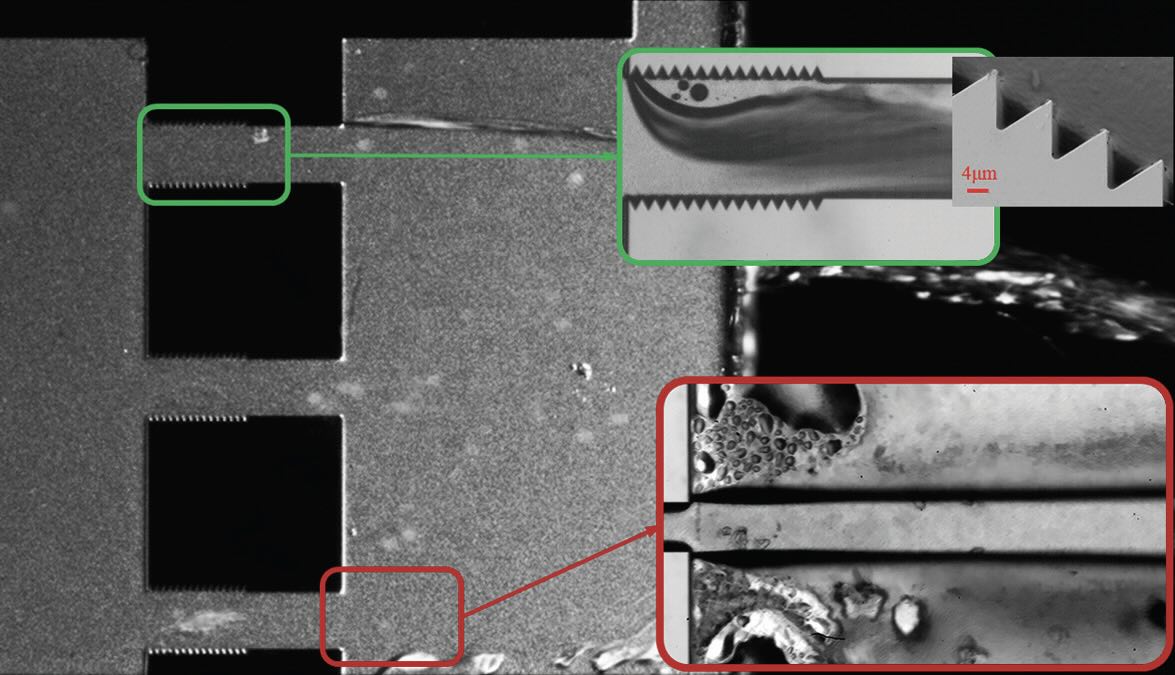
Figure 4. An image of the actual reactor used that was able to reduce levels of one specific PFAS in pilot studies by 55%. Figure courtesy of Oxford Brooks University.
The Stockholm wastewater was evaluated for PFAS, and 11 substances (PFAS11) were identified at low parts per trillion (nanograms per liter) levels. Included among the PFAS were two well-known members, PFOA and PFOS. Detection of the PFAS was conducted using high-performance liquid chromatography-Tandem mass spectrometry.
Three reactors were designed and fabricated to evaluate the effectiveness of developed cavitating flows in targeting PFAS. Parameters such as size, length and roughness elements on the sidewall were varied among the reactors. Reactors 1 and 2 contained single microchannels with the difference being the first reactor has a roughened surface while the second reactor has a smooth surface. A third reactor had a multi-channel configuration with sidewall roughness elements placed within the microchannels. All of the reactors were effective in degrading all PFAS11 even at low concentrations (approaching levels close to those in drinking water).
Tzanakis says, “During hydrodynamic cavitation, the generated microbubbles collapse violently, releasing powerful shock waves that can cause local spikes in pressure (up to the gigapascal range) and temperature (over 1,000°C). The degree of sidewall roughness affects the cavitation dynamics and hence which PFAS degrade more readily. For example, Reactor 1 displays the best performance of all reactors in degrading PFOS and PFNA (perfluorononanoic acid). Degradation rates of 55% and 45% were achieved, respectively.”
The researchers speculate that hydroxyl radicals generated during the process play a crucial role in the collapse of cavitation bubbles, but further research is required to support the hypothesis. As this process is occurring, the hydroxyl radicals initiate cleavage of some carbon-fluorine bonds present in the PFAS through interactions with C1 fluoro radicals. Further work needs to be done to determine the mechanism and identify the byproducts formed.
The success of this approach on a pilot scale will lead to scaling hydrodynamic cavitation to a commercial level. Ghorbani says, “We will next evaluate PFAS containing wastewater at the 20 liter level. If successful, we hope to evaluate hydrodynamic cavitation in a wastewater treatment plant in Stockholm.”
Tzanakis says, “The simplicity of this approach means that PFAS degradation can take place not just in an aqueous medium but also potentially in mineral oil base stocks. Our intention in the future is to determine if hydrodynamic cavitation can be used to degrade PFAS in a Group II base stock. If successful, this means that hydrodynamic cavitation could be used as a technique to remove PFAS from lubricants.”
Additional information can be found in a recent article
2 or by contacting Ghorbani at
morteza.ghorbani@sabanciuniv.edu or Tzanakis at
itzanakis@brookes.ac.uk.
REFERENCES
1.
Canter, N. (2024), “PFAS: New approach for decomposition using microbes,” TLT,
80 (11), pp. 12-13. Available
here.
2.
Talabazar, F., Baresel, C., Ghorbani, R., Tzanakis, I., Kosa, A., Grishenkov, D. and Ghorbani, M. (2024), “Removal of per- and polyfluroalkyl substances (PFAS) from wastewater using the hydrodynamic cavitation on a chip concept,”
Chemical Engineering Journal, 495, 153573.