Atomism
By Tyler Housel, Contributing Editor | TLT Lubrication Fundamentals February 2025
The physical sciences, including tribology, generally seek to answer two basic questions: What are things made of? How do things change over time?
One interesting observation about these two basic questions is that we organize matter into discrete atomic units, but change is viewed as a continuous process. Throughout history, philosophers conceptualized the building blocks of matter in many ways including both discrete and continuous theories. The debate was finally settled in the early 20th century1 when the modern concept of the atom emerged with enough predictive evidence to convince the scientific community that atoms were real. This atomistic style of thinking is explicitly used in chemistry and also is found in other disciplines that may not seem obvious at first.
Let’s imagine we are somewhere in Central America thousands of years ago. Flo and Jake are preparing squash, maize, chiles and beans when a caveman returns to the camp. After discussing the merits of insurance, the conversation turns to dinner. We notice that the food items are all different but also share some similarities. The squash is mostly water and the chiles obviously have fire inside, but they all provide sustenance that does not exist in the soil, water or air. We wonder if the earth, air and water absorb fire from the sun to make food. Then the fire in the food sustains us when we eat. Could everything in our world be simply combinations of these four basic things?
Although modern atomic thinking has added nuance to this ancient wisdom, we still learn that the everyday states of matter are solids, liquids and gases with plasma as a fourth phantasmagorical category. This sounds a lot like air, earth, fire and water, so Empedocles was on the right track (see Table 1).
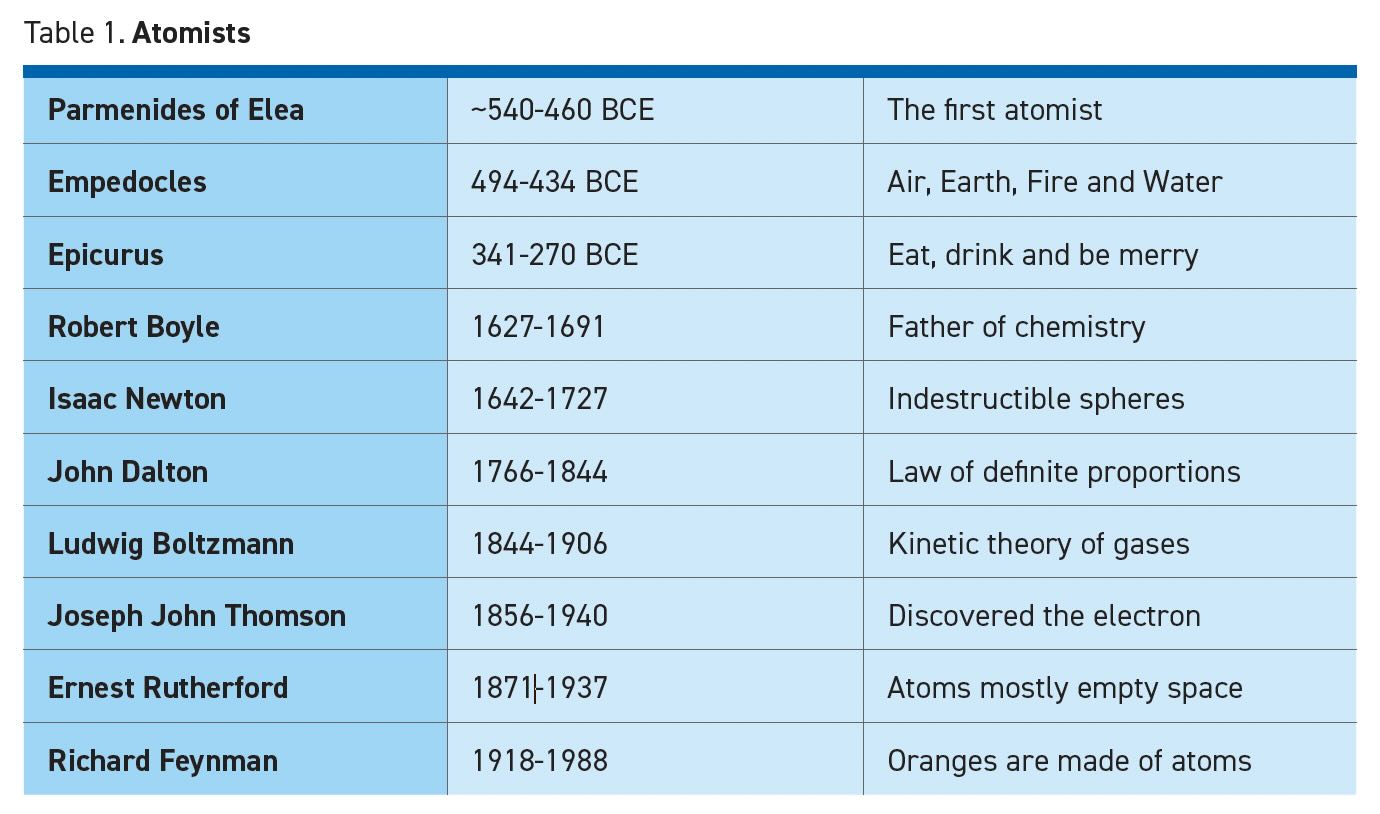
In the fifth century BCE, Greek philosophers debated the nature of the physical world, and the two prevailing theories were substance metaphysics and process metaphysics. Parmenides is considered the father of atomism because his cryptic writings posited that everything is built from changeless elementary atoms which have innate properties—“atom” coming from “atomos” meaning cannot be cut. Parmenides explained the diversity of the world as a consequence of the many possible combinations and ratios of these atoms.
Chemists associate atomism with carbon, oxygen and other chemical elements. More broadly, atomism breaks nature down into fundamental units that lose essential properties if we attempt to divide them further. This harkens back to Socrates (through Plato’s writings) who suggested that the objective of natural philosophy was to carve nature at its joints.2 An anatomist3 could consider a bone as an element in the skeleton and the joint marks the natural place to distinguish one bone from another. To a tribologist, an element could be the ball in a bearing and a biologist may think of each ant as an element in an anthill.
Atomistic models are based on a collection of real things. Heraclitus offered a rival theory that reality was ultimately a web of rule-based processes without the need for material substances (see Table 2). Even though atoms are now regarded as real, process methodology is also alive and well. It is widely used in physics and engineering when dealing with fields or bulk systems where the population is large enough to disregard the properties of individual atoms. Some examples include electromagnetics, stress/strain analysis, friction, economics, optics and many more. Scientists may use either atomistic or continuous approaches depending on the situation.
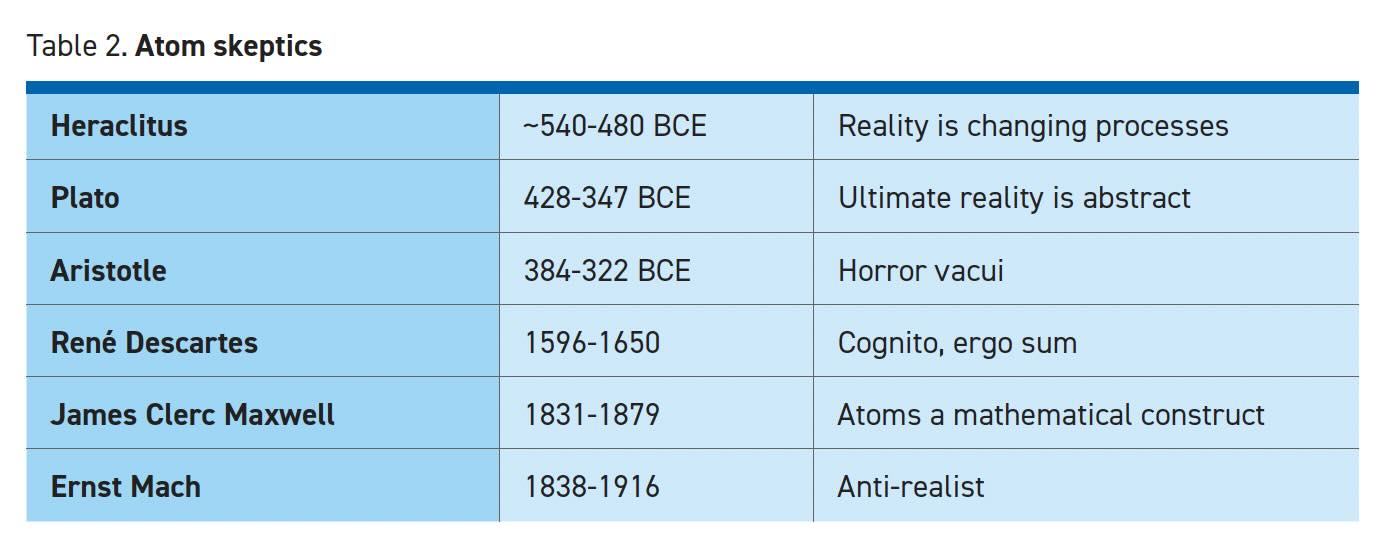
For every Plato, who argued that ultimate reality consists of Forms such as Goodness and Beauty,4 there was an Epicurus who used atomism to champion his moral philosophy that opposed religion, reasoning that everything is made of atoms whose only enduring properties are size, shape, weight and motion. After death, the atoms simply rearrange into a new pattern without moral consequence. The Epicurean philosophy has come to stand for the pursuit of immediate, material gratification.
Another challenge to atomism dates back to Aristotle’s belief that vacuum could not exist in nature. We are all familiar with the saying “nature abhors a vacuum” or “horror vacui” in Latin. If something fills all space, then it would be impossible for an atom to exist separate from the whole. René Descartes5 explicitly denied that space could be separated from matter and therefore matter must be a continuous substance. Of course, Descartes also famously opined that reality could not be separated from the mind: “I think therefore I am,” or “cognito, ergo sum.”
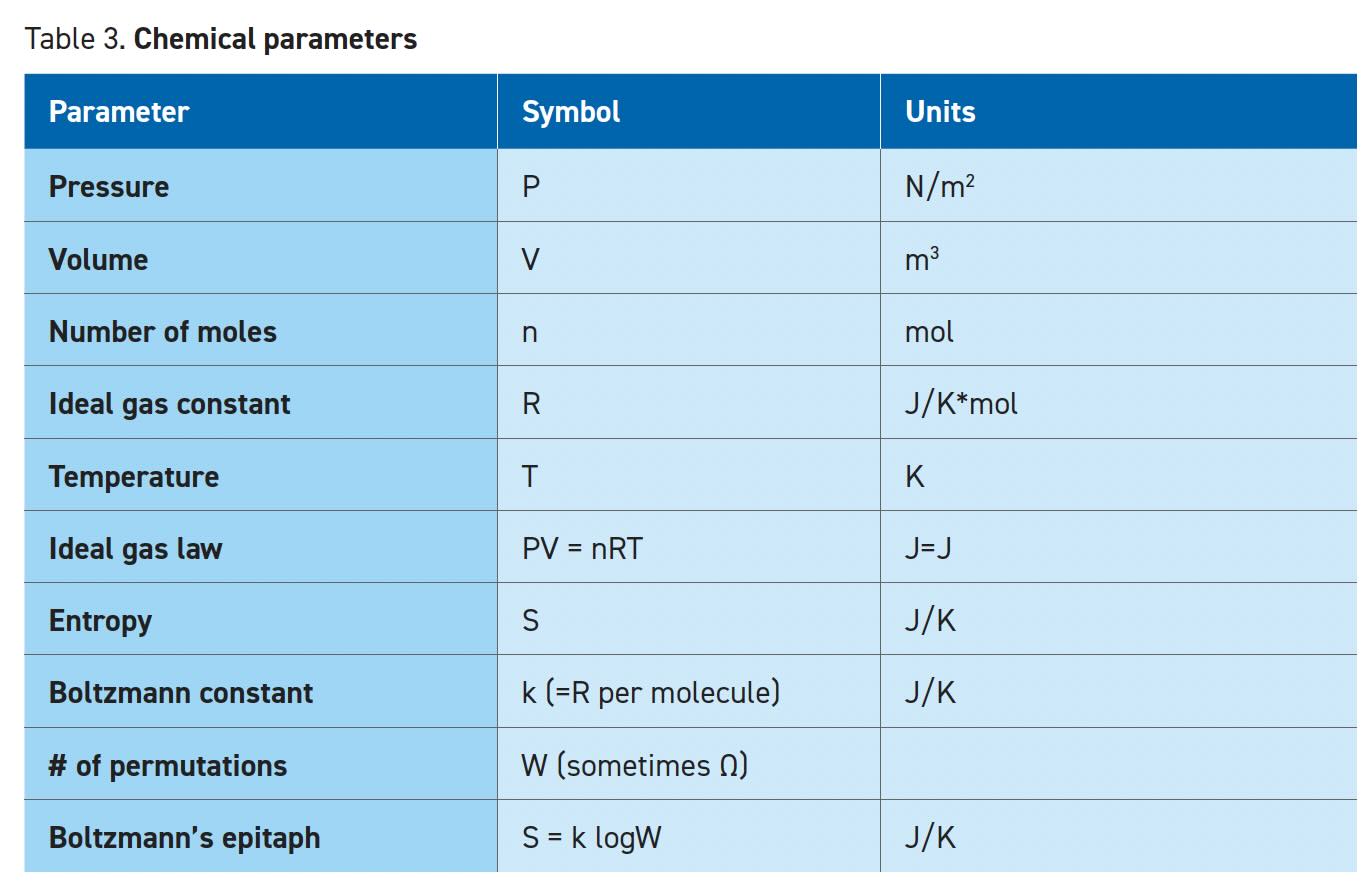
Robert Boyle,6 the “father of chemistry,” was an early advocate of the belief that chemistry involves the reactions of a limited number of distinct atoms. Boyle’s law of gases states that pressure times volume is a constant value and was eventually combined with other gas laws to become the familiar ideal gas law (PV = nRT, see Table 3).
Isaac Newton proposed that matter consists of minute, indestructible spheres animated by forces of attraction and repulsion. He also wrote that light was atomic (corpuscular) because a prism can separate individual colors, but each color remains true when reflected or refracted.
As chemists such as Boyle, Sir Humphry Davy, Antoine Lavoisier and others began to identify chemical elements, John Dalton7 concluded that all atoms of a particular element are identical. All chemistry could be explained by transformations of these fixed elements which can be separated or joined in different fixed ratios to form new compounds. Dalton’s belief in ratios and proportions was accepted, but prominent scientists still believed these were mathematical relationships and rejected the idea that actual atomic particles existed.
Ludwig Boltzmann relied on the behavior of atoms (or small molecules) to develop statistical mechanics and the kinetic theory of gases. His equation S = k log W (see Table 3) describes the relationship between entropy and the number of possible atomic states. The Boltzmann constant “k” is the gas constant “R” for individual ideal gas atoms. Lest anyone forget who developed the equation for entropy, it is printed on Boltzmann’s gravestone in Vienna (see Figure 1). Ernst Mach8 agreed with Boltzmann’s use of atomic theory as a mathematical tool, but his anti-realist philosophy left him as the last major scientist who stated in 1910 that he was “in no great hurry to take them (atoms) for realities.”

Figure 1. The equation for entropy is printed on Boltzmann’s gravestone in Vienna. Figure courtesy of Feldkurat Katz, CC BY-SA 4.0, via Wikimedia Commons.
Even as atomic thinking took hold, it became apparent that the atom was not a uniform unbreakable entity as Newton and others surmised. J.J. Thomson found that electrons could exist outside the atom, so atoms could be divided into positively and negatively charged parts. Soon thereafter, Ernest Rutherford’s gold foil experiments led to the conclusion that the atom had an incredibly dense nucleus surrounded by mostly empty space. The next step inward was the discovery that the nucleus was made of protons and neutrons. And then these turned out to be composed of quarks, leptons and bosons. Particle physicists now have several species in the subatomic particle zoo (see Figure 2).9
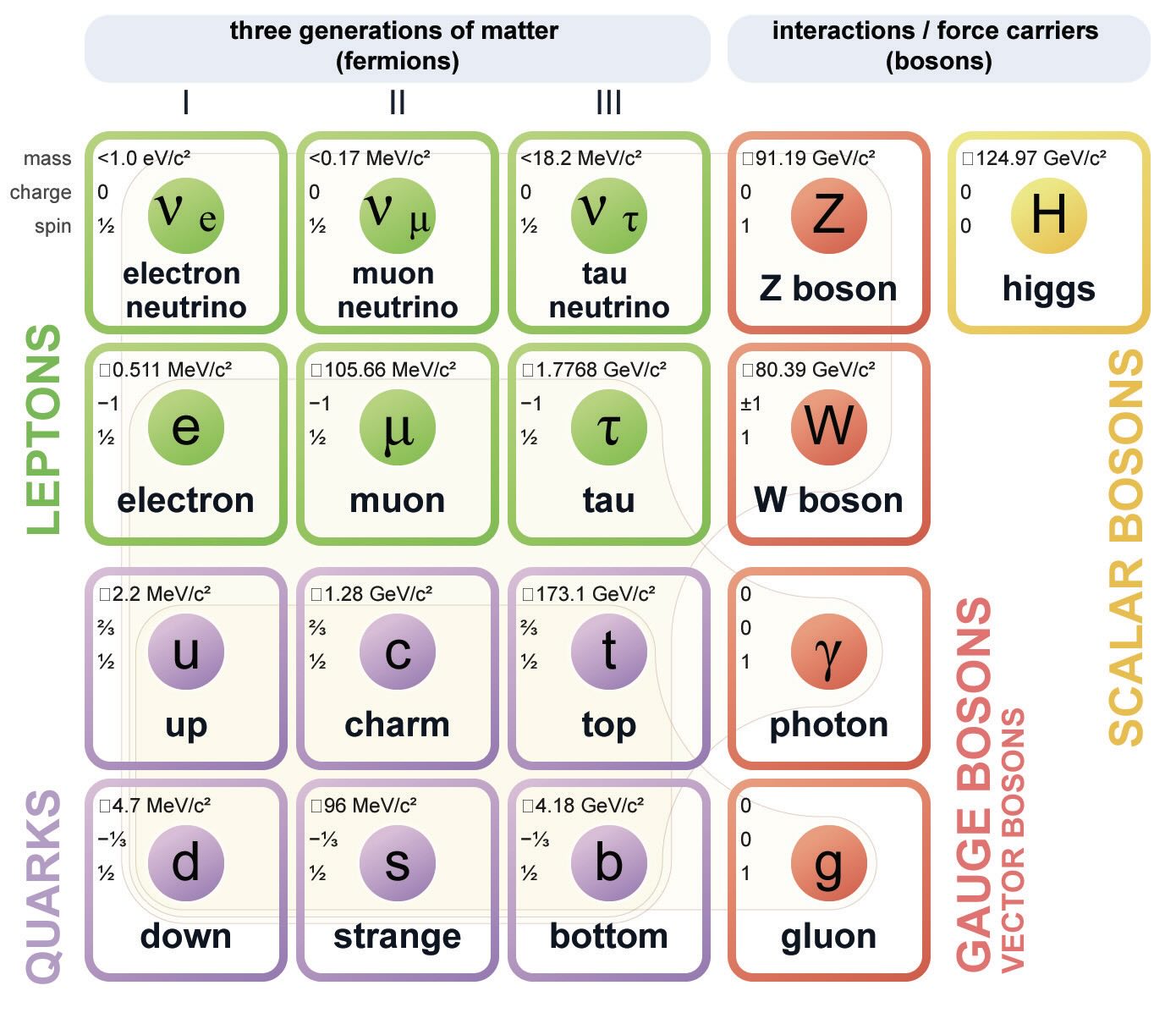
Figure 2. Standard model of elementary particles. Figure courtesy of MissMJ, Cush, minor edit by Acrux13, CC BY 3.0, via Wikimedia Commons.
Until tribologists start to work with big atom smashers, we can breeze through the last paragraph. Readers of TLT will find that the Periodic Table of the Elements is a box of 98 crayons that has all the colors needed to draw a tribologist’s world, so the answer to the first question is that things are made of atoms (see Figure 3). This leaves the second question: How do things change over time?
Figure 3. Periodic Table of the Elements.
Since antiquity, philosophers wondered whether matter was continuous or discrete. But time and space were always assumed to be continuous. If we believe that atoms exist, is it also possible that space is broken into discontinuous pieces? Actually, some quantum theories speculate that space is divided into tiny pixels that are around 10-35 meters in length.10 This is billions of billions of times smaller than anything we can measure experimentally, so no one knows if it’s true. It may eventually affect our thinking on black holes but won’t impact the results of a four-ball wear test.
Why is it important to understand the difference between discrete and continuous explanations of the natural world? Matter, time, space and motion all appear continuous to our senses. We are accustomed to a world where motion is continuous and are easily fooled into seeing motion when viewing a sequence of still pictures with a smooth transition of action and a time interval of at most 0.05 seconds (20 frames per second). Further, the still pictures themselves are a discrete pattern of small pixels of light which are seen by a discrete number of photoreceptors in the eye. Despite the gaps within and between the images, our brain interprets this as continuous motion.
Tribology studies material objects (discontinuous) in relative motion (continuous) and uses calculus to formulate a mathematical description of this motion which eventually leads to an understanding of acceleration, forces, fields, energy, power and other dynamic properties. Differential calculus allows us to calculate the instantaneous rate of change of an equation at any point in time but only if the equation is a continuous function. Therefore, calculus implicitly assumes that time and space are continuous variables; it is not possible to differentiate individual atoms (see Atomism Debate).11
Atomism debate
The 20th century physicist and philosopher Richard Feynman used atomism to debate his mathematician colleagues in graduate school:
They (math geeks) would explain to me, “You’ve got an orange. OK? Now you cut the orange into a finite number of pieces, put it back together, and it’s as big as the sun. True or false?”
“Impossible! There ain’t no such a thing.”
“Ha! We got him… it’s So and so’s theorem of immeasurable measure!”
Just when they think they’ve got me, I remind them, “But you said an orange! You can’t cut the orange peel any thinner than the atoms.”
“But we have the condition of continuity. We can keep on cutting.”
“No, you said an orange.” So I won.A
A. Feynman, R.P. (1985), Surely You’re Joking, Mr. Feynman! New York City, N.Y.: Bantam Books.
Apologies to the math geeks, but an example may help: a car going from STLE headquarters in Chicago to the STLE Annual Meeting in Atlanta, May 18-22, will travel 1,248 km (775 miles12). If the journey takes 12 hours, the average speed is 104 km/hour, which is the distance traveled (∆x) divided by the time (∆t). A simple algebraic solution is sufficient to calculate the average speed. But the police are more interested in the car’s speed when we pass their radar gun. Like an analog speedometer, calculus divides the instantaneous change in distance (dx) by the instantaneous change in time (dt) to give a continuous readout of the car’s speed. This is only possible if both x (distance) and t (time) are continuous variables, and the journey can be represented as an infinite series of infinitely small time steps.
∆t=t1-t0 (finite step)
dt=lim (t1-t0 ) (infinitely small steps)
(∆t→0)
Yes, math geeks only apply calculus to continuous functions, but engineering nerds routinely use digital instruments which have a finite sampling rate (∆t). As long as ∆t is small enough to map the points to a continuous function, the geeks and nerds shake hands and say, “Close enough for the marketing goobers.”
Why has the atomistic model emerged as the best fit for physical reality? The answer lies in the emergent properties which show up when dividing the world down to the atomic level. Carving nature at its joints produces a network of relationships between time, space and atoms. Understanding these relationships, chemists can reconstruct nature in endless epicurean combinations.
REFERENCES
1. Goldman, S.L. (2007), Great Scientific Ideas That Changed the World. Chantilly, VA: The Teaching Company.
2. Bird, A. and Tobin, E. (Spring 2024 Edition), “Natural Kinds.” Available here.
3. Anatomist contains the word atomist, but actually derives from “ana” (up) and “tomos” (cut). Available here.
4. https://plato.stanford.edu/entries/plato/
5. https://plato.stanford.edu/entries/descartes/
6. https://www.britannica.com/biography/Robert-Boyle
7. https://en.wikipedia.org/wiki/Atomism
8. https://plato.stanford.edu/entries/ernst-mach/
9. www.animatedphysics.com/particle_zoo/wiki_standard_model.jpg
10. Interested readers can look this up under “Plank Length” named after Max Plank.
11. Calculus can be used to estimate the properties of sufficiently large collections of atoms.
12. Note: Miles are listed for the benefit of STLE President Jack McKenna.
Tyler Housel is a technologist for Hnuco Technologies and is based in Lansdale, Pa. You can reach him at tylerhousel@comcast.net.