HIGHLIGHTS
•
A flash-within-flash (FWF) joule heating process was used to synthesize the solid lubricant molybdenum diselenide.
•
Evaluation of the molybdenum diselenide produced from FWF joule heating with commercially available material was conducted using a ball-on-flat tribometer.
•
Molybdenum diselenide produced by FWF joule heating displayed a superior coefficient of friction value probably due to its higher crystalline content with no evidence of an amorphous layer.
Solid lubricants are utilized in severe applications where conventional liquid-based counterparts do not provide sufficient thermal and oxidative stability to meet the specific operating requirements. One example of a solid lubricant used in tribology applications is molybdenum diselenide.
The traditional method for producing molybdenum diselenide is to react the two elements (molybdenum and selenium) in a sealed tube at elevated temperatures (> 600°C). Additional processing is required under high temperatures and low pressures to purify this solid lubricant making it suitable for use. The net result is synthesis of molybdenum diselenide, and it is energy-intensive and time consuming.
In a previous TLT column,
1 a technique known as flash joule heating was able to convert waste plastic into two materials, hydrogen and graphene, that are finding growing uses in sustainable applications. Flash joule heating of a readily available plastic, high-density polyethylene, involved placing the material in quartz tube with carbon black in the presence of copper wool and graphite rod electrodes. The starting materials were compressed to achieve a resistance between 5-10 Ohm, and the mixture was subjected to a 100-130 volt discharge for less than three seconds. Running four consecutive flash joule experiments led to a yield of hydrogen up to an efficiency of 92.7%. Graphene and other hydrocarbons were found in the residue of the reaction mixture. From a sustainability standpoint, a life cycle assessment found that hydrogen was produced at a much lower cost than the U.S. Department of Energy goal of $1 per kilogram.
To expand the versatility of this sustainable pathway, a modified technique known as flash-within-flash (FWF) joule heating process has now been developed that can produce solid lubricants in gram scale quantities. James Tour, T.T. and W.F. Chao Professor of Chemistry and professor of materials science and nanoengineering at Rice University in Houston, Texas, says, “In FWF, two quartz vessels are oriented in a concentric manner. The outer vessel contains an inexpensive conductive feedstock such as metallurgical coke, while the starting reagents (molybdenum and selenium in the case of molybdenum diselenide) are placed in an inner-semi-closed reactor.”
Upon generation of a current through a custom-made capacitor banks discharge system, resistive joule heating produces temperatures in the range of 2,000°C, which is then transferred by thermal conduction to the inner tube resulting in a high yield of molybdenum diselenide after a reaction duration of only five seconds.
Tour says, “We moved to FWF to overcome the conductivity limitations of flash joule heating. This method allows us to synthesize a wider number of materials such as molybdenum diselenide that can be prepared more economically and sustainably.”
Figure 2 shows the progression from flash joule heating to using FWF joule heating.
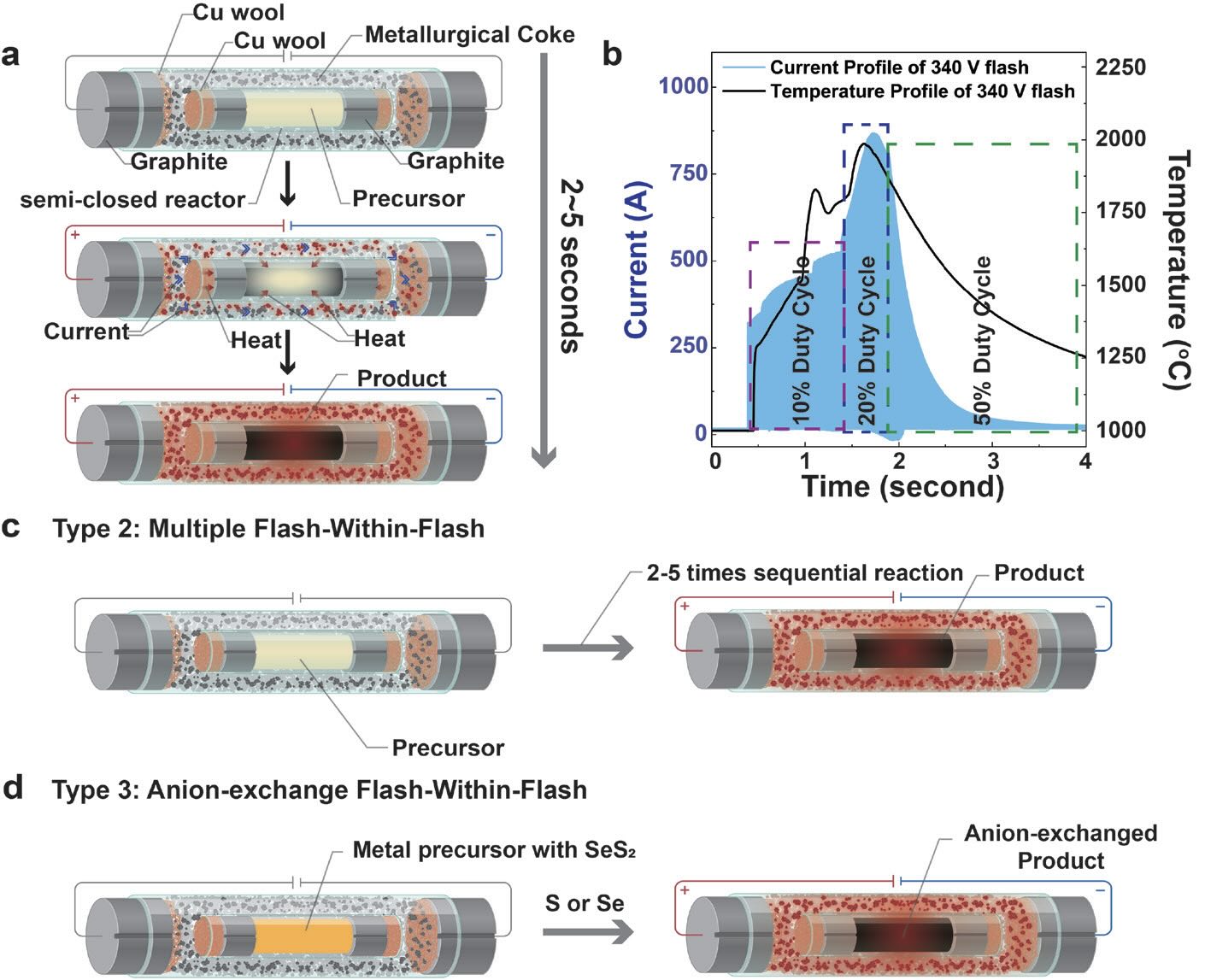 Figure 2. The concept of flash joule heating has evolved from originally producing graphene and hydrogen (a) to developing flash-within-flash (b) and to finally using the FWF joule heating technique to produce molybdenum diselenide. Figure courtesy of Rice University.
Figure 2. The concept of flash joule heating has evolved from originally producing graphene and hydrogen (a) to developing flash-within-flash (b) and to finally using the FWF joule heating technique to produce molybdenum diselenide. Figure courtesy of Rice University.
The next step was for the tribological properties of FWF molybdenum diselenide to be evaluated versus a sample prepared by conventional means.
Ball-on-flat tribometer
STLE member C. Fred Higgs III, John and Ann Doerr Professor of Mechanical Engineering at Rice University, worked with Dr. Victoria Granja, a postdoctoral researcher from his Particle Flow & Tribology Lab at Rice University, to conduct a comparative analysis of the coefficient of friction for FWF prepared versus commercially sourced molybdenum diselenide. Higgs says, “The FWF process presents the possibility for a greater potential to use molybdenum diselenide in commercial applications. Our approach was to first understand if there is a tribological benefit (e.g., low friction) using FWF and then determine the reason for this benefit.”
The researchers used a ball-on-flat tribometer in the testing. Granja says, “We applied molybdenum diselenide powder to a rectangular flat alumina sample. A 52100 chrome steel ball was then slid against the coated alumina in a reciprocating motion under 1, 5 and 10 newton loads at a frequency of 3 Hz for 10 minutes. The total mass of the coating was approximately 0.2 gram.”
After two minutes of testing, a noticeable difference in the coefficient of friction for the two molybdenum diselenide samples was observed. The FWF materials displayed coefficient of friction reductions of 69%, 20% and 41% at the applied loads of 1, 5 and 10 newtons, respectively. Granja says, “We noticed that the film for the commercially available molybdenum diselenide started to degrade at a faster rate as the test-approached 10 minutes.”
Coefficient of friction versus time curves in Figure 3 for the two types of molybdenum diselenide and for alumina demonstrate the potential for using FWF joule heating.
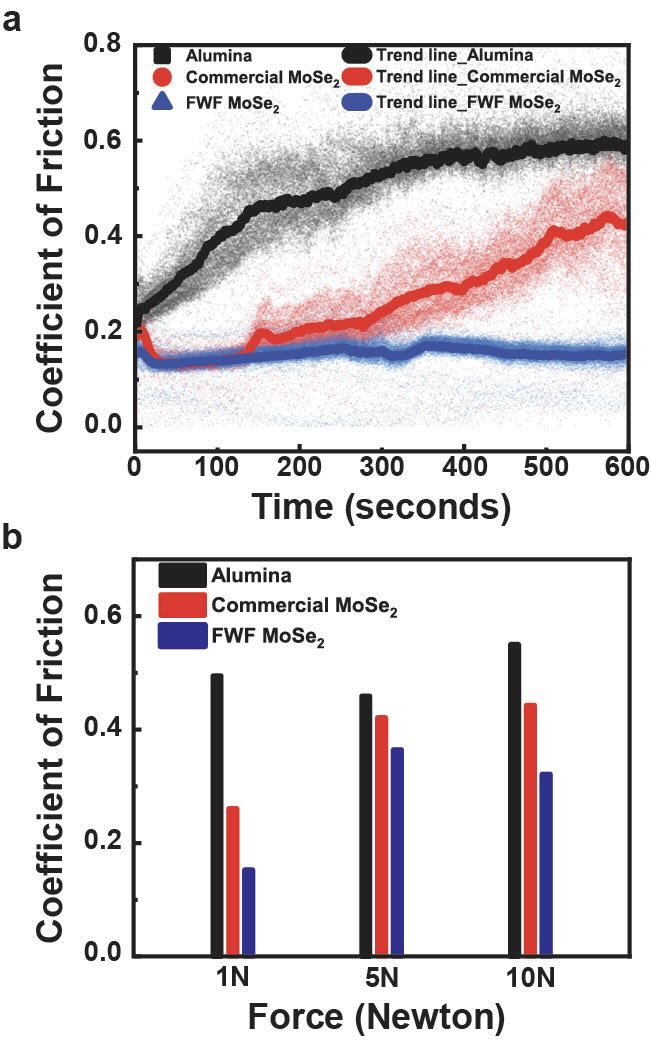 Figure 3. Coefficient of friction curves versus time (a) and force (b) demonstrate the benefits of FWF joule heating produced molybdenum diselenide versus a commercially available grade of this solid lubricant. Figure courtesy of Rice University.
Figure 3. Coefficient of friction curves versus time (a) and force (b) demonstrate the benefits of FWF joule heating produced molybdenum diselenide versus a commercially available grade of this solid lubricant. Figure courtesy of Rice University.
With help from Chi Hun Choi, a doctoral student in the material science and nanoengineering department, this result prompted the researchers to conduct a scanning transmission electron microscopy study on the original powders to determine if there are differences in their composition that might explain the coefficient of friction findings. Granja says, “The commercial molybdenum diselenide exhibited an estimated 10 micron thick amorphous layer at its edges. In contrast, the FWF produced material contained well-defined crystalline edges with no evidence of amorphous content. The crystallinity enabled the FWF molybdenum diselenide to slide very easily leading to lower coefficient of friction values at the loads used in the tribometer study.”
Higgs indicates that other solid lubricants will be prepared and evaluated using FWF. He says, “Two candidates are molybdenum disulfide and tungsten disulfide. These are both lamellar materials.”
Higgs is convinced that FWF is a unique process that has the potential to be commercially scaled up. He adds, “My hope is that FWF can be used to prepare newer materials that may have unique multifunctional properties to complement the presently available solid lubricants.”
Additional information can be found in a recent article.
2 Questions about the FWF process can be addressed to Tour at
tour@rice.edu. Further information on the tribology study can be obtained by contacting Higgs at
higgs@rice.edu.
REFERENCES
1.
Canter, N. (2023), “Production of hydrogen and graphene from waste plastic,” TLT,
79 (12), pp. 18-19. Available at
www.stle.org/files/TLTArchives/2023/12_December/Tech_Beat_II.aspx.
2.
Choi, C., Shin, J., Eddy, L, Granja, V., Wyss, K., Damasceno, B., Guo, H., Gao, G., Zhao, Y., Higgs, C., Han, Y. and Tour, J. (2024), “Flash-within-flash synthesis of gram-scale solid-state materials,”
Nature Chemistry, 16, pp. 1831-1837.