HIGHLIGHTS
•
Converting waste streams, such as cow manure, into a carbon-rich material known as biochar leads to the production of hydrogen through the electrochemical oxidation process.
•
Bubbling argon or nitrogen through the anolyte produced a frothy, bubbling mixture that created the ideal environment for electrochemical oxidation at the anode.
•
Hydrogen generation was assisted by higher carbon-to-oxygen ratio at the surface of biochar particles and the higher negative zeta potential.
Ongoing research to find more efficient approaches for producing hydrogen from renewable sources has focused on techniques for splitting water. One significant hurdle to overcome is the high amount of voltage needed in the oxygen evolution reaction that occurs at the anode.
Dr. Meenesh Singh, associate professor of chemical engineering at the University of Illinois, Chicago in Chicago, Ill., says, “The oxygen evolution reaction suffers from sluggish kinetics leading to the need for a theoretical equilibrium potential of 1.23 V versus a reversible hydrogen electrode, plus an overpotential of at least 0.2 V, to generate high current densities. Empirical studies demonstrated that the lowest cell potential (sum of equilibrium potential and overpotential) is between 1.5 and 1.6 V. This can produce one to three amperes of current per square centimeter. The question to be asked is: Can a better approach be found to reduce the cell potential?”
A past TLT article
1 highlighted a unique method for splitting hydrogen through the use of metal chips derived from titanium, nickel and stainless steel alloys. These chips can be generated during metal removal operations and are considered to be a waste stream. Inclusion of two known metal catalysts, platinum and cobalt, to the titanium and nickel alloys, improved water splitting efficiency. Oxygen evolution reaction performance was found to occur when a small amount of cobalt was added to the nickel alloy. A current density of 40 milliamps per square centimeter was achieved at a cell potential of 1.6 V versus a reversible hydrogen electrode.
Singh realized through a literature search that electrochemical oxidation of carbon at the anode may offer a more attractive option. He says, “Carbon can react with water at the anode to produce carbon dioxide and hydrogen cations (i.e., protons). Theoretically, the process can be done at a significantly lower equilibrium potential of 0.21 V versus the standard hydrogen electrode. Using coal as the carbon source afforded a potential as low as 0.15 V versus the standard hydrogen electrode.”
The accompanying hydrogen evolution reaction at the cathode means that the overall process produces both carbon dioxide and hydrogen, separately. While splitting of water producing green hydrogen through electrolysis has potential as a sustainable process, it makes sense to identify a suitable carbon source that is renewable.
One concern with this approach is how to handle carbon dioxide, which is recognized as a greenhouse gas. Singh believes that this material can either be converted into a useful derivative or captured and subsequently used to regenerate biomass so that it can be reused as the carbon source in splitting water.
Singh and his colleagues have now identified a series of substrates derived from biomass that can now be utilized in the electrochemical oxidation reaction.
Biochar
The researchers sourced several different waste streams that are derived from biomass. These include agricultural waste (e.g., wheat straw, corn stover and rice husk), municipal waste (e.g., solid waste and sludge from wastewater treatment plants) and animal waste (e.g., cow, sheep and horse manure).
All of these waste streams were converted to a carbon-rich material known as biochar
(see Figure 5) that serves as the substrate for the oxygen evolution reaction. Singh says, “We decided to convert the waste streams to biochar using a low-temperature charring-sulfonation method. This approach enabled us to produce biochar in a slurry form which can be directly used in the electrochemical oxidation process. Sulfuric acid acts as the catalyst in producing biochar and also is the anolyte for producing carbon dioxide at a platinized titanium mesh anode. The same electrolyte is also used as the catholyte with a platinum cathode to facilitate production of hydrogen.”
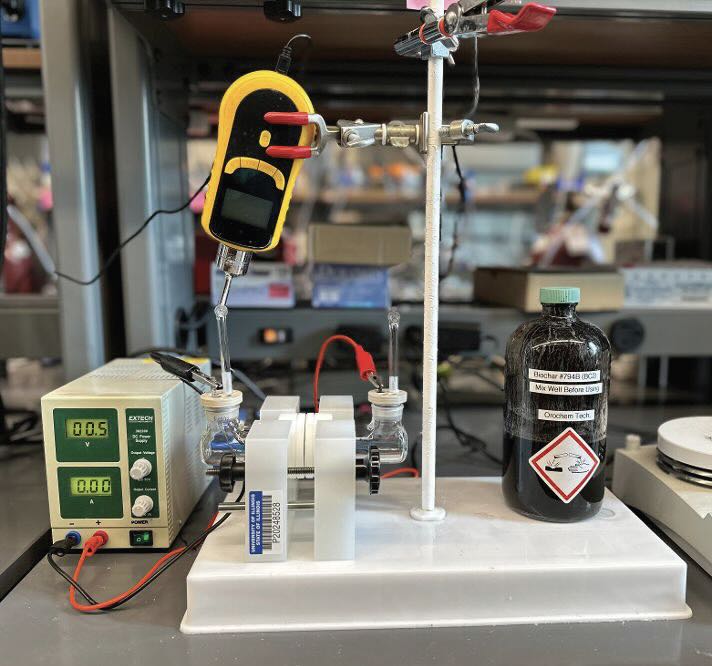 Figure 5. A carbon-rich material derived from renewable sources and known as biochar (shown in the bottle on the right of the image) can be used directly in electrochemical oxidation to produce hydrogen. Figure courtesy of the University of Illinois, Chicago.
Figure 5. A carbon-rich material derived from renewable sources and known as biochar (shown in the bottle on the right of the image) can be used directly in electrochemical oxidation to produce hydrogen. Figure courtesy of the University of Illinois, Chicago.
A sulfonated tetrafluoroethylene-based fluoropolymer is used as the membrane separating the anolyte from the catholyte. The experimental setup is shown in Figure 6 and the process is known as biochar-assisted water electrolysis (BAWE).
 Figure 6. The experimental setup used to convert renewable waste streams into hydrogen is shown. Figure courtesy of the University of Illinois, Chicago.
Figure 6. The experimental setup used to convert renewable waste streams into hydrogen is shown. Figure courtesy of the University of Illinois, Chicago.
The problem faced by the researchers was finding a way to maximize the contact between particles from the biochar source and the anode. Singh says, “In initial experiments, we did not detect any current at cell potentials below 1.25 V. But bubbling argon or nitrogen through the anolyte led to the creation of a frothy, bubbling mixture that created the ideal environment for electrochemical oxidation at the anode.”
The most effective biomass source was found to be cow manure. Singh says, “We determined that generating biochar from cow manure can produce current down to a cell potential of 0.2 V. The reason for cow manure’s effectiveness is due to the properties of the biochar including a lower moisture content, optimum volatile matter, lower particle size, higher average pore diameter and higher level of crystallinity compared to biochar from other biomass waste streams. Running the process at 80°C maximized hydrogen production.”
Two other factors that assist with hydrogen generation are the higher carbon-to-oxygen ratio at the surface of the biochar particles and the higher negative zeta potential. As the former increases, the current density generated as hydrogen is formed also increases.
Singh says, “We used a silicon solar cell as the green energy source to power the process. Overall efficiency was found to be approximately 35%.”
One problem faced by the researchers was the biochar particles produced tend to be encapsulated in a layer of residual ash that formed during the electrochemical oxidation and poisoned the particles. Singh indicated that steps will be taken to remove the ash. He adds, “We will be working to improve the reaction stability so that 100% consumption will be achieved. Commercializing this novel electrochemical approach will be challenging as new electrolyzers will need to be developed to stabilize the carbon source in a slurry as it is transported.”
Additional information can be found in a recent article
2 or by contacting Singh at
mrsingh@uic.edu.
REFERENCES
1.
Canter, N. (2024), “Hydrogen production using metal chips,” TLT,
80 (8), pp. 12-13. Available at
www.stle.org/files/TLTArchives/2024/08_August/Tech_Beat_I.aspx.
2.
Kani, N., Chauhan, R., Olusegun, S., Sharan, I., Katiyar, A., House, D., Lee, S., Jairamsingh, A., Bhawnani, R., Choi, D., Nielander, A., Jaramillo, T., Lee, H., Oroskar, A., Srivastava, V, Sinah, S., Gauthier, J. and Singh, M. (2024), “Sub-volt conversion of activated biochar and water for H2 production near equilibrium via biochar-assisted water electrolysis,”
Cell Reports Physical Science, 5 (6), 102013.