Hydrogen embrittlement can cause an alloy to lose ductility, which can limit the metal’s ability to act as a structural material.
An experimental study was conducted to better understand the effect hydrogen has on nickel-based alloy Inconel 725 at four different electrochemical charging levels.
Metal ductility declined and the number of cracks observed increased with higher levels of hydrogen exposure.
The movement toward decarbonization is leading to a growing use of hydrogen. As this transition occurs, research is ongoing to determine how to produce hydrogen from renewable sources cost effectively and then figure out an efficient manner to store it so it is readily available when needed.
A past TLT article
1 highlighted work underway to store hydrogen underground so that it can be extracted in a similar manner to natural gas. Testing was conducted by exposing hydrogen to shale and to sandstone. Both were obtained from known geological formations. Hydrogen was found to display better adsorption in shale due to this material’s smaller pore size compared to sandstone.
Challenges are emerging in working with hydrogen including how this gas may interact in a negative fashion with metal alloys. The result can lead to a phenomenon known as hydrogen embrittlement. Dr. Michael Demkowicz, professor in the Department of Materials Science and Engineering at Texas A&M University in College Station, Texas, says, “Hydrogen embrittlement can cause an alloy to lose ductility which can limit the metal’s ability to act as a structural material. But there is considerable uncertainty about whether exposure to hydrogen will impact a metal alloy. In some cases, the metal alloy remains unaffected.”
Demkowicz indicates that titanium and zirconium-based alloys are more susceptible to hydrogen embrittlement because both metals react with hydrogen to form hydrides. Steel and nickel-based alloys are less vulnerable to hydrogen embrittlement. The former is more likely to be used because of cost, but there are applications where a nickel alloy may be required due to its superior mechanical properties.
Demkowicz says, “Hydrogen embrittlement can occur in nickel-based alloys which will reduce their toughness to that of aluminum alloys. One of the problems is determining how quickly metal ductility declines. This continues to be very unpredictable and the mechanism for hydrogen embrittlement has not been ascertained.”
Demkowicz’s research has focused on evaluating nickel-based alloys. Two hypothesized mechanism, hydrogen-enhanced localized plasticity (HELP) and hydrogen-enhanced decohesion (HEDE) have emerged to potentially explain hydrogen embrittlement in nickel alloys. The former proposes that hydrogen enhances localized plasticity that promotes metal fracture while the latter claims that hydrogen weakens atomic bonds.
Demkowicz and his colleagues conducted an experimental study to better understand the effect increasing levels of hydrogen have on the structural integrity of the high-strength, corrosion resistant, nickel-based alloy Inconel 725.
Crack initiation
The researchers introduced hydrogen at four different electrochemical charging levels into flaw-free Inconel 725 specimens with identical composition and microstructure. Samples were charged for approximately 50 hours at the following current densities: 1.5, 5 and 25 milliamps per square centimeter. The fourth sample was a control that was not exposed to hydrogen. Under this method of charging, hydrogen was not uniformly distributed but concentrated in layers along the surfaces.
Demkowicz says, “Hydrogen was concentrated into approximately 50 micron thick surface layers immediately after charging with peak concentrations ranging from 1,870 to 5,120 atomic parts per million, progressing from the low- to the high-hydrogen content samples. The center of the Inconel 725 samples was hydrogen-free.”
In situ tensile strength testing was performed on all samples at room temperature in a vacuum chamber. This procedure was conducted in a scanning electron microscope to facilitate studying the metal surface through the use of scanning electron microscopy. The average strain rate was 4 x 10
-5 per second over times of four hours or less. Approximately 44% of the charged hydrogen was lost from outgassing and did not interact with the metal specimens.
Demkowicz says, “As the hydrogen content in the sample increases, tensile testing showed that elongation to failure decreases as expected from metal samples subjected to hydrogen exposure. The medium hydrogen content samples displayed surface and immediate subsurface cleavage-type fracture while the midsection contains dimples
(see Figure 5). This means that the near-surface region was subjected to hydrogen embrittlement, but the interior of the metal sample was not.”
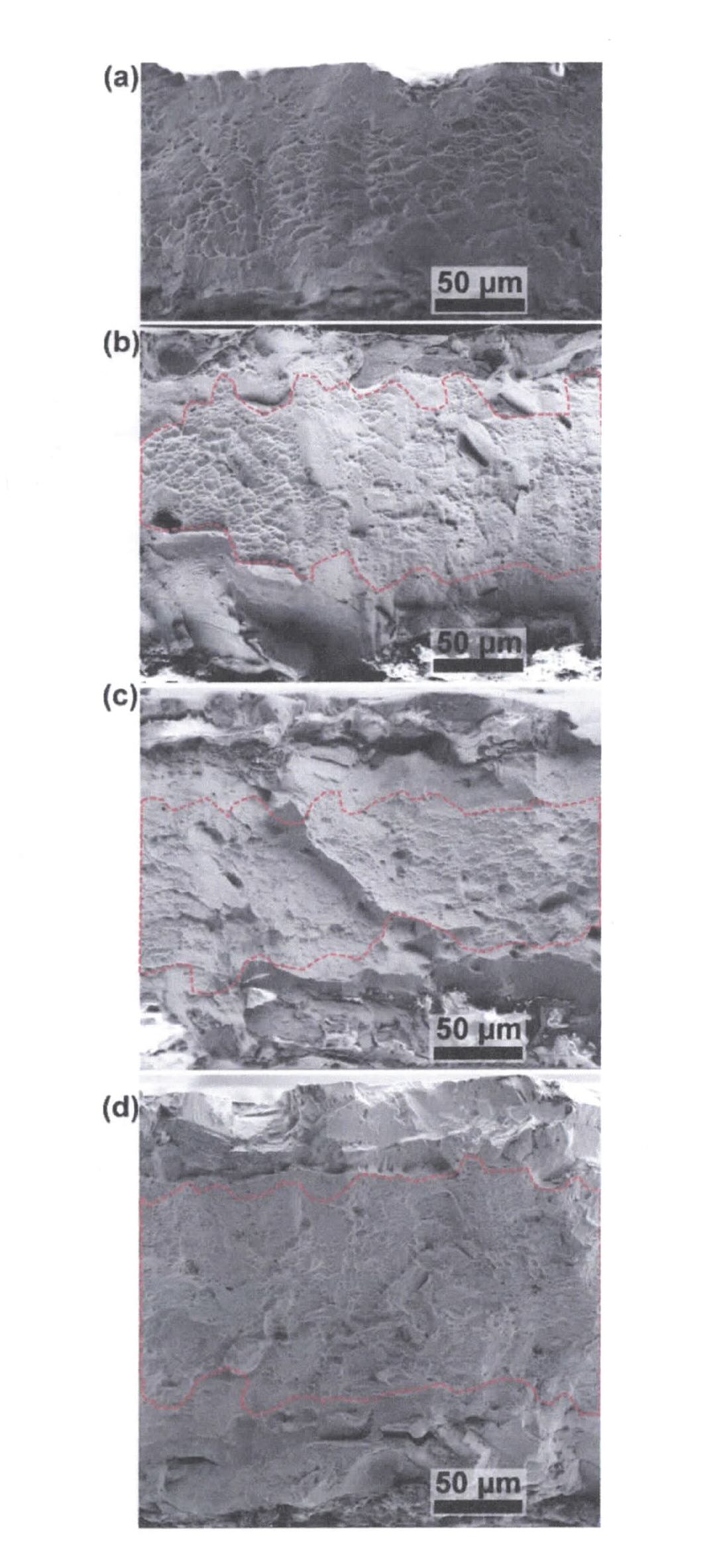 Figure 5. SEM micrograph of fracture surfaces. (a) H-free sample, (b) low H sample, (c) medium H sample and (d) high H sample. The H-free sample exhibits a dimpled fracture surface along the entire sample cross section. All three samples charged with H show a transition from cleavage brittle fracture near the surface to dimpled ductile fracture in the interior. Red dashed lines mark the transition between cleavage and dimpled regions. Image of each individual sample is stitched from multiple focusing points to show the full thickness of fracture surface with high resolution. Reprinted with permission of AAAS from Science Advances 10 (29), DOI: 10.1126/sciadv.ado2118, © Liu, M., Jiang, L. and Demkowicz, M. some rights reserved; exclusive licensee AAAS. Distributed under a CC BY-NC 4.0 License (http://creative commons.org/licenses/by-nc/4.0/).
Figure 5. SEM micrograph of fracture surfaces. (a) H-free sample, (b) low H sample, (c) medium H sample and (d) high H sample. The H-free sample exhibits a dimpled fracture surface along the entire sample cross section. All three samples charged with H show a transition from cleavage brittle fracture near the surface to dimpled ductile fracture in the interior. Red dashed lines mark the transition between cleavage and dimpled regions. Image of each individual sample is stitched from multiple focusing points to show the full thickness of fracture surface with high resolution. Reprinted with permission of AAAS from Science Advances 10 (29), DOI: 10.1126/sciadv.ado2118, © Liu, M., Jiang, L. and Demkowicz, M. some rights reserved; exclusive licensee AAAS. Distributed under a CC BY-NC 4.0 License (http://creative commons.org/licenses/by-nc/4.0/).
The number of cracks observed by the researchers increased with the increase in hydrogen content. Cracks in the metal surface are an indication of embrittlement leading to loss of mechanical properties such as ductility. Past publications proposed that cracks tend to occur near areas of slip in the metal.
Demkowicz says, “Slip is an indication of plasticity in the metal and is a mechanism describing deformation. Dislocations gliding on a single crystal metal plane produce slips. We observed localized deformation in Inconel 725 samples exposed to hydrogen that are characterized as slip bands. Somc cracks initiate near regions of high slip. However, as hydrogen content increases, so do the number of cracks initiated at locations where there is no slip.”
This work shows that the HELP mechanism probably does not explain hydrogen-induced crack initiation in Inconel 725 due to cracks initiating in regions without nearby slip in the presence of high levels of hydrogen. Demkowicz says, “Questions still remain whether the HEDE mechanism is responsible for hydrogen cracks propagating after they have initiated or could there be another mechanism?”
Future work will concern studying hydrogen embrittlement in other alloys to determine if the same type of phenomenon is observed, and to gain a better understanding of how hydrogen interacts with the metal surface and the mechanism for crack propagation.
Additional information can be found in a recent article
2 or by contacting Demkowicz at
demkowicz@tamu.edu.
REFERENCES
1.
Canter, N. (2024), “Potential for storing hydrogen underground,” TLT,
80 (8), pp. 16-17. Available at
www.stle.org/files/TLTArchives/2024/08_August/Tech_Beat_III.aspx.
2.
Liu, M., Jiang, L. and Demkowicz, M. (20240, “Role of slip in hydrogen-assisted crack initiation in Ni-based alloy 725,”
Science Advances, 10 (29), DOI: 10.1126/sciadv.ado2118.