An anode-free sodium solid-state battery was prepared that contains an electrochemically stable electrolyte, has intimate contact between the solid electrolyte and current collector, a dense solid-state electrolyte and a highly dense current collector.
The battery contained a sodium borohydride solid-state electrolyte known as NBH that displayed good stability and performance in the presence of sodium.
Pelletized aluminum was used to ensure that the there is a strong solid-solid interfacial contact between the solid electrolyte and the aluminum foil based current collector.
The battery is probably the most important component for use in applications such as electric vehicles. Lithium-ion batteries became the leading technology because of their high energy density. But issues such as supply chain and cost are leading researchers to evaluate other options.
Sodium batteries have emerged as a potential option even though they do not exhibit as high an energy density as their lithium counterparts. But sodium is more readily available and lower in cost.
In a past TLT article,
1 a new potential electrolyte for use in sodium-ion batteries was discussed. The electrolyte consisted of sodium bis(fluorosulfonyl) in a mixture of dimethyl carbonate and tris (2,2,2,-trifluoroethyl) phosphate. In long-term cycling testing, this electrolyte achieved greater than 90% retention after more than 300 cycles. Improved ionic conductivity and low interfacial resistance were observed at different charge/discharge rates. An additional benefit was that the solid electrolyte interface (SEI) formed was more stable because its components were not as readily compatible with the electrolyte.
One approach for developing a sodium battery that may be able to exhibit comparable performance to a lithium-ion battery is to remove the anode. Y. Shirley Meng, professor of molecular engineering in the University of Chicago’ Pritzker School of Molecular Engineering, says, “Producing an anode-free sodium battery is appealing for two reasons. The first is developing a battery without an anode simplifies the manufacturing process. Without the presence of an anode, electrochemical deposition of sodium ions on the surface of the current collector is required for the battery to operate. This second reason is higher volumetric energy density are no possible leading to higher cell voltages, reducing costs and accelerating battery charging.”
One of the main barriers to removing the anode is to replace the organic liquid electrolytes commonly used in batteries with a solid-state alternative. Meng says, “Using liquid electrolytes is not feasible because a SEI forms that will react with battery ions reducing effectiveness. Moving to a solid-state electrolyte is a better strategy though the challenge is to modify the sodium battery architecture. To achieve this objective, scaling up the battery architecture so that it can be commercialized is essential.”
Research in developing solid-state electrolytes has focused on lithium-ion batteries. Unfortunately, the resulting batteries have failed to display sufficient reversible cycling or added too much inactive material to the cell.
Meng reveals that an anode-free sodium solid-state battery must meet four conditions. She says, “An electrochemically stable or highly passivating electrolyte is needed, intimate contact between the solid electrolyte and current collector is required, a dense-solid-state electrolyte must be utilized, and the current collector also needs to be highly dense.”
Meng states that all four of these conditions must be satisfied simultaneously. These conditions have now been met with the development of the world’s first anode-free sodium solid-state battery.
Sodium borohydride solid electrolyte
The researchers initially prepared an anode-free sodium solid-state battery that included a commonly used solid electrolyte, Na
3PS
4, and an aluminum foil current collector. Unfortunately, the initial Coulombic efficiency was only 4% because of the reduction of NaPS
4 at low potential.
To improve performance, the researchers identified a sodium borohydride material known as NBH (Na
4B
10H
10B
12H
12) as a suitable solid-state electrolyte because this caged substance is stable in the presence of sodium metal. Substitution of NBH with aluminum foil produced a much higher initial Coulombic efficiency of 64%, which while better, was still not acceptable for commercialization.
Meng says, “We were surprised about the stability and performance of NBH in the presence of sodium. NBH displayed good performance under controlled experimental conditions with a dew point of -60°C
(see Figure 3).”
 Figure 3. The experimental setup used to evaluate the anode-free sodium solid-state battery is shown. Figure courtesy of David Baillot/University of California San Diego Jacobs School of Engineering.
Figure 3. The experimental setup used to evaluate the anode-free sodium solid-state battery is shown. Figure courtesy of David Baillot/University of California San Diego Jacobs School of Engineering.
X-ray diffraction analysis showed that inadequate solid-solid interfacial contact between the NBH solid electrolyte and the aluminum foil current collector explained the inferior performance. To address this issue, the researchers discovered that pressing pelletized aluminum onto the solid electrolyte separator during cell construction produced an initial Coulombic efficiency of 93% due to the uniform sodium metal distribution on the current collector surface.
Meng says, “The presence of pelletized aluminum led to an increase in metal density on the current collector. Critical current density also increased to 6.0 milliamps per square centimeter, which is due to improved sodium plating uniformity where the metal is distributed over a larger area.”
Working with a dense solid electrolyte and dense current collector are critical to ensure battery performance. As shown in Figure 4c, a dense solid electrolyte produces intimate interface contact with the current collector and avoids dendrite growth that can destabilize a battery. A dense current collector ensures that no sodium ions can be trapped within the current collector. It that were to occur, battery performance would significantly suffer.”
Meng adds, “Dendrite formation can be very harmful, and this phenomenon appears to be even worse in sodium as compared to lithium batteries. NBH is a solid electrolyte that displays amorphous characteristics with no grain boundaries making it ideal for sodium batteries.”
Once the battery is produced, a key concern is stack pressure. Meng says, “Stack pressure is the ‘Achilles Heel’ for solid-state batteries. Sufficient pressure must be maintained to minimize the presence of empty gaps in the electrolyte. This can be accomplished through mechanical means, but additional engineering work is needed to develop packaging for the sodium battery that will apply the needed stack pressure.”
The researchers prepared an anode-free sodium solid-state full cell using sodium chromium oxide as the cathode combined with a catholyte that is based on sodium yttrium, zirconium and chlorine. Cycling at 40°C produced initial Coulombic efficiency of 93%. The cell remained stable for 400 cycles with a capacity retention of 70% and an average Coulombic efficiency of 99.96%.
As a result, the researchers satisfied the four conditions for an anode-free sodium solid-state battery. This included a feasible anode-free configuration
(see Figure 4a) and a dense anode-free material
(see Figure 4b).
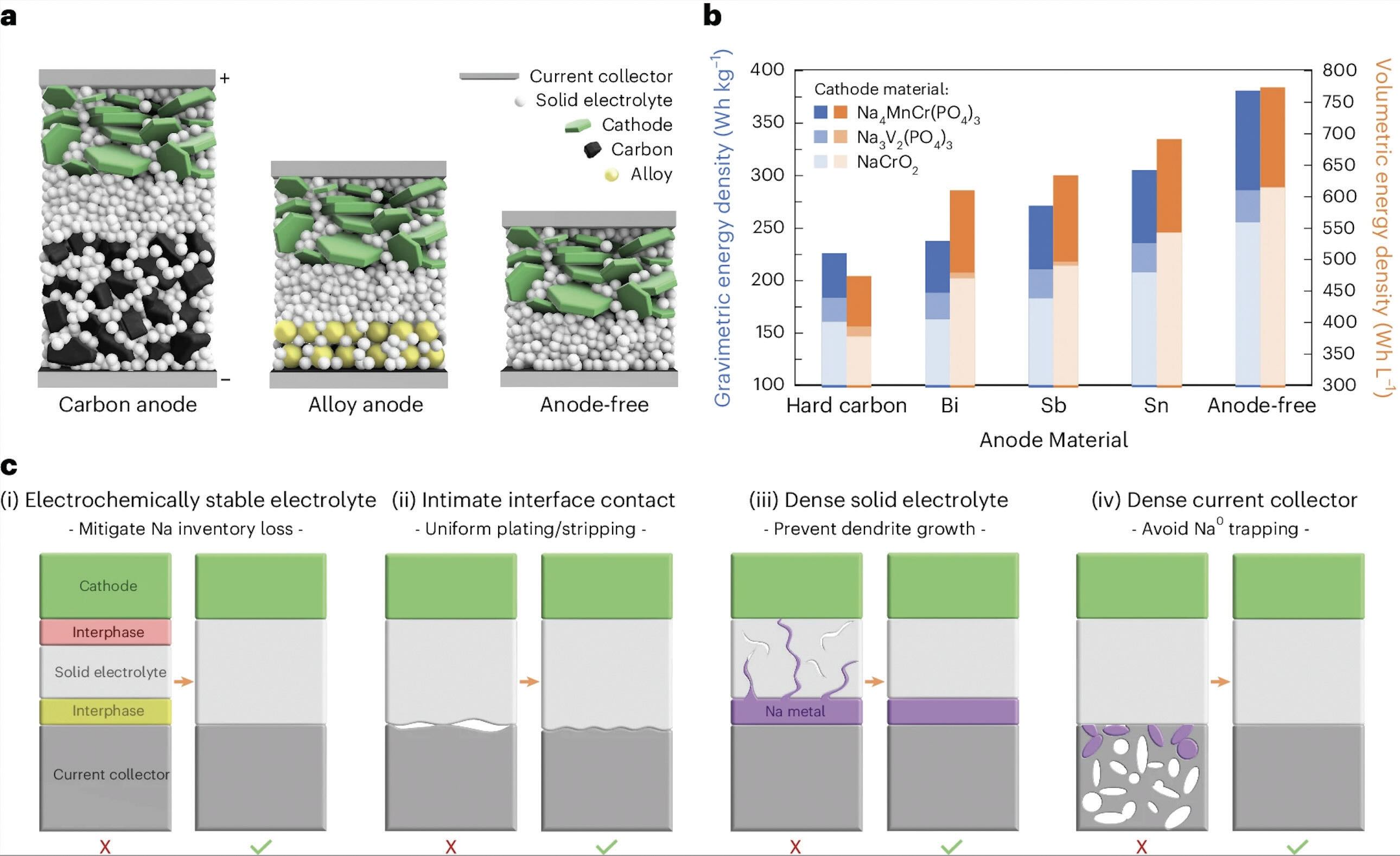 Figure 4. (a) Schematics illustrate the configuration for carbon anode, alloy anode and anode-free sodium batteries. (b) A theoretical energy density analysis demonstrates that an anode-free configuration can produce superior performance versus four anode materials. (c) The need for a properly configured solid electrolyte and solid current collector is essential to avoid dendrite formation in the former and the trapping of sodium in the latter. Figure courtesy of the University of Chicago.
Figure 4. (a) Schematics illustrate the configuration for carbon anode, alloy anode and anode-free sodium batteries. (b) A theoretical energy density analysis demonstrates that an anode-free configuration can produce superior performance versus four anode materials. (c) The need for a properly configured solid electrolyte and solid current collector is essential to avoid dendrite formation in the former and the trapping of sodium in the latter. Figure courtesy of the University of Chicago.
Meng says, “We are evaluating a new cathode and new catholyte. Chromium-based materials are not desired because commercial users are concerned about the possibility of chromium (VI), a carcinogen, formed during use. Instead, our approach will be to work with alternative sodium phosphates as the cathodes.”
The researchers also intend to scale up the technology and produce a pouch cell. Meng indicates that work needs to be done to boost the conductivity three to five times to make the sodium battery.
Additional information on this research can be found in a recent paper
2 or by contacting Meng at
shirleymeng@uchicago.edu.
REFERENCES
1.
Canter, N. (2022), “New potential electrolyte for sodium-ion batteries,” TLT,
78 (11), pp. 16-17. Available at
www.stle.org/files/TLTArchives/2022/11_November/Tech_Beat_II.aspx.
2.
Deysher, G., Oh, J., Chen, Y., Sayahpour, B., Ham, S., Cheng, D., Ridley, P, Cronk, A., Lin, S., Qian, K., Nguyen, L., Jang, J. and Meng, Y. (2024), “Design principles for enabling an anode-free sodium all-solid-state battery,”
Nature Energy, 9, pp. 1161-1172.