HIGHLIGHTS
•
A series of metal dopants were added to a copper catalyst to assess their influence on the electrochemical carbon dioxide reduction reaction.
•
Metals displaying stronger oxygen affinity such as rhodium weaken the carbon-oxygen bond of carbon dioxide leading to the formation of hydrocarbons such as ethylene.
•
Gold is an example of a metal that exhibits weaker oxygen affinity that, when used in doping the copper catalyst, sends the reaction pathway to alcohol formation.
Multiple approaches are underway to determine how to effectively convert carbon dioxide electrochemically into hydrocarbons such as ethylene and alcohols such as ethanol. One of the challenges has been to find an appropriate catalyst system that will convert carbon dioxide, a very stable molecule with a linear structure, into an electrochemically active species.
A past TLT article
1 used a functional ionic liquid in combination with a copper catalyst to facilitate conversion of carbon dioxide electrochemically to formate, succinate, methane, ethylene and ethane. Carbon dioxide was found to interact with the ionic liquid above the surface of a copper electrode. The reaction yield increased as the concentration of ionic liquid was boosted. But the presence of a thickened double layer structure above the copper catalyst finally caused the reaction to slow due to steric constraints.
Jingjie Wu, associate professor in the department of chemical and environmental engineering at the University of Cincinnati in Cincinnati, Ohio, says, “Copper catalysts have two limitations when used in the carbon dioxide electrochemical reduction reaction. The first is selectivity because currently a distribution of products with one, two and three carbons is formed from carbon dioxide. A second challenge is to synthesize hydrocarbon derivatives with carbon chains greater than three. It will be very desirable to produce a molecule with eight to 10 carbons that could be converted into a lubricant base stock intermediate. But the electrocatalytic reduction reaction can only proceed at temperatures at or above ambient. It is hard to operate an electrochemical cell at a temperature above 100 °C, which may be the only current way to form higher molecular weight hydrocarbons.”
Wu points out that past work has shown the carbon dioxide electrochemical reduction reaction pathway may proceed through a selectivity-determining intermediate. Once this species forms, the reaction reaches a fork where one path produces hydrocarbons while a second one leads to alcohol formation.
Wu says, “The issue of a selectivity-determining intermediate has been a matter of a long-standing debate among researchers. Unfortunately, such a species probably only exists for a short period of time. This means that identifying and isolating the intermediate with any current tool or technology is probably extremely difficult.”
One strategy for improving product selectivity is to regulate the carbon-oxygen bond strength of the selectivity-determining intermediate. Wu says, “A catalyst that promotes weak carbon-oxygen bond strength will shift the product distribution to hydrocarbons. If the carbon-oxygen bond is stronger, then the oxygen will remain with the product leading to higher percentage of alcohol formation.”
Wu and his colleagues have now determined that adding metal dopants to the copper catalyst can impact carbon-oxygen bond strength leading to an improvement in product selectivity.
Rhodium-doped copper catalyst
Prior to empirically synthesizing metal-doped copper catalysts, the researchers conducted a density functional theory modeling study to identify candidates based on considering the selectivity-determining intermediate to be an acetaldehyde species. The following noble metal dopants were evaluated in this phase of the study: rhodium, iridium, ruthenium, palladium, silver and gold.
Wu says, “These metals were selected because of their thermodynamic stability in a copper matrix. Three of these metals (rhodium, iridium and ruthenium) work with the copper catalyst to create catalyst sites that stabilize oxygen species weakening the carbon-oxygen bond and leading to hydrocarbon formation. The other three metals (palladium, silver and gold) exhibit weaker oxygen affinity sending the reaction pathway toward alcohol formation.”
Based on this study, metal-doped copper catalysts were synthesized by cation exchange. In the case of rhodium, copper hydroxide was reacted with rhodium trichloride. The rhodium-doped copper catalyst was then produced through a calcining treatment.
Evaluation of the performance of the rhodium-doped catalyst was conducted using a flow cell with one molar potassium hydroxide as the electrolyte
(see Figure 5). Wu says, “The rhodium-doped copper catalyst achieved a conversion of 70% to ethylene in recent work we conducted. This represents a 50% increase in ethylene selectivity.”
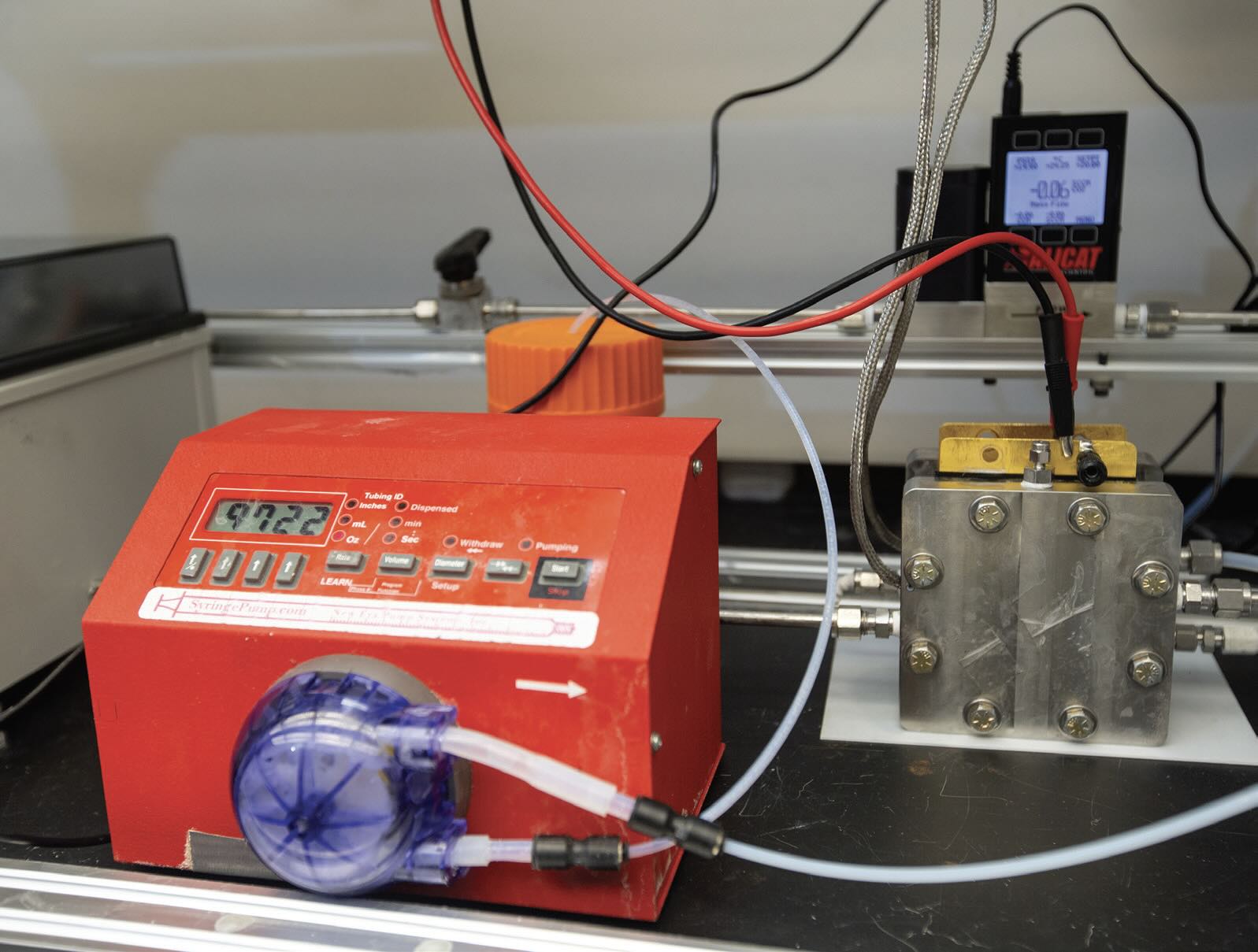 Figure 5. This flow cell was used to evaluate the performance of a rhodium-doped copper catalyst in the electrochemical carbon dioxide reduction reaction. Figure courtesy of the University of Cincinnati.
Figure 5. This flow cell was used to evaluate the performance of a rhodium-doped copper catalyst in the electrochemical carbon dioxide reduction reaction. Figure courtesy of the University of Cincinnati.
In evaluating the other metal-doped catalysts, the researchers found that iridium and ruthenium doping also yielded selectivity for ethylene formation compared to copper. The other three metal-doped catalysts (palladium, silver and gold) exhibited less selectivity than copper.
The stability of the rhodium-doped copper catalyst was determined at a potential of -0.66 V versus a reversible hydrogen electrode, where the best selectivity was realized. The catalyst sustained its performance for at least 35 hours. Wu says, “Future work will be needed to extend the performance of the catalyst so that the process can become commercially viable. Currently a big challenge is to overcome the instability found in the gas diffused electrode being used. Our goal is to have the catalyst operate effectively for at least several thousand hours.”
Wu also is looking to develop catalyst systems that will produce longer chain hydrocarbons in the future. He says, “Our work shows that affinity for oxygen at the catalyst surface may be an approach to predicting selectivity in the electrochemical carbon dioxide reduction reaction. Oxygen bound selective-determining intermediates such as acetaldehyde probably are involved in this process, though we cannot rule out the possibility of other species influencing the reaction.”
Additional information can be found in a recent article
2 or by contacting Wu at
jingjie.wu@uc.edu.
REFERENCES
1.
Canter, N. (2024), “Multifunctional ionic liquid: A co-catalyst for electrochemical carbon dioxide reduction reaction,” TLT,
80 (4), pp. 16-17. Available
here.
2.
Li, Z., Wang, P., Lyu, X., Kondapalli, V., Xiang, S., Jimenez, J., Ma, L., Ito, T., Zhang, T., Raj, J., Fang, Y., Bai, Y., Li, J., Serov, A., Shanov, V., Frenkel, A., Senanayake, S., Yang, S., Senftle, T. and Wu, J. (2024), “Directing CO
2 electroreduction pathways, for selective C
2 product formation using single-site doped copper catalysts,”
Nature Chemical Engineering, 1 (2), pp. 159-169.