TLT: How did you start working in tribology?
Viat: I was finishing my master’s thesis on mechanical behavior of the tomato’s cell wall and asked my lab colleagues if they had plans to apply for a doctoral contract. The offer was titled “Study of tribocorrosion damage at the CMC blade/disk contact subjected to fretting wear at high temperatures” and promised to investigate a very precise aeronautical problem. At that time, I needed to search three words in this doctoral offer before applying! After my doctoral defense, I started as a lubrication engineer at Constellium. This was six years ago. I am no longer dealing with fretting wear at Constellium, but it is still a pleasure to edit articles about fretting for Tribology Transactions journal. Fretting wear is a rather active field of research, more than lubrication for aluminum rolling apparently! Sadly I haven’t found the time to publish again, this time in the field of metalworking fluids.
TLT: What makes your current job so fascinating?
Viat: I am a tribologist for a metallurgical company, which means I don’t have many colleagues who are familiar with concepts like hydrodynamic lubrication or stick-slip. So my job at Constellium is to be the tribology’s agent and account for the knowledge related to tribology in the company. To do so, I need to learn both from the inside and outside. Inside, it is needed to understand and formalize the tribology issues from colleagues. Outside is where I can learn from the worldwide tribology community to educate myself and try to fulfill my colleagues’ needs in tribology. I rely a lot on TLT and other STLE resources to improve my skills in tribology!
Tribology is a discipline studying interfaces. Similarly, my job is at the interface between Constellium’s proper expertise in rolling aluminum products and the external tribological expertise required to optimize lubrication. That makes my job fascinating for sure, because I never stop learning from both sides! Moreover, I enjoy considerable freedom to choose what I can do to contribute to lubrication expertise at Constellium. Many of my topics are related to complex industrial issues where it is difficult to say clearly that lubrication is to blame. So my day-to-day activities include devising experiments to answer these questions: “What is the tribological cause of this industrial issue?” and “What needs to be done to improve the situation?” This implies spending a lot of time in the lab to perform reduced-scale tests. This is not far from how I worked when I was a doctorate student! Except that I now have far more interactions with colleagues and responsibilities in delivering adapted, on-time solutions.
TLT: What is emulsion stability, and how can it be determined?
Viat: An emulsion is a dispersion of a liquid A in a liquid B with which liquid A is not miscible. The liquid A is called dispersed phase and the liquid B is the continuous phase. This is a metastable system because the two liquids tend to separate; the mixture destabilizes. Emulsion stability represents how long the system will keep its integrity over time. Emulsions can be kinetically stable, with no visible destabilization over time, but remain thermodynamically unstable and sensitive to any change of the ambient conditions.
The simplest way to determine emulsion stability is to prepare the emulsion in a transparent vessel, wait and visually assess stability after a given time. The stability measurement becomes quantitative if the top and bottom parts of the destabilized emulsion are sampled and analyzed (e.g., concentration of liquid A in liquid B for top and bottom: emulsion stability index [ESI]). To replace the naked eye and also automatically obtain the destabilization kinetics, emulsion stability can be analyzed through static multiple light scattering (SMLS); an incident light beam interacts with the emulsion sample. The backscattered light is captured on a receptor. Repeating the quantification of backscattered light over a certain period of time enables us to obtain the destabilization kinetics.
Analyzing the particle size distribution (PSD) of liquid A in liquid B also is an indirect mean to determine emulsion stability. Some emulsions destabilize through droplet size increase, which becomes a parameter to follow. PSD analysis can be performed through light diffractometry or electrical conductivity (Coulter counter). Another technique to be mentioned is flow imaging, where pictures of the emulsion are taken through a microscope and further analyzed to determine the PSD of the emulsion.
Other techniques exist to characterize dispersed micro-objects, like small-angle neutron scattering (SANS), but I am not aware of any use case in metalworking fluids. The PSD-related techniques have the advantage of being dynamic; an emulsion flow is analyzed in a similar state to the emulsion in use. On the contrary, SMLS or ESI techniques describe the destabilizing emulsion at static state, which gives a different information. From my experience, however, the static and dynamic means to determine the stability correlate rather well for a given emulsion.
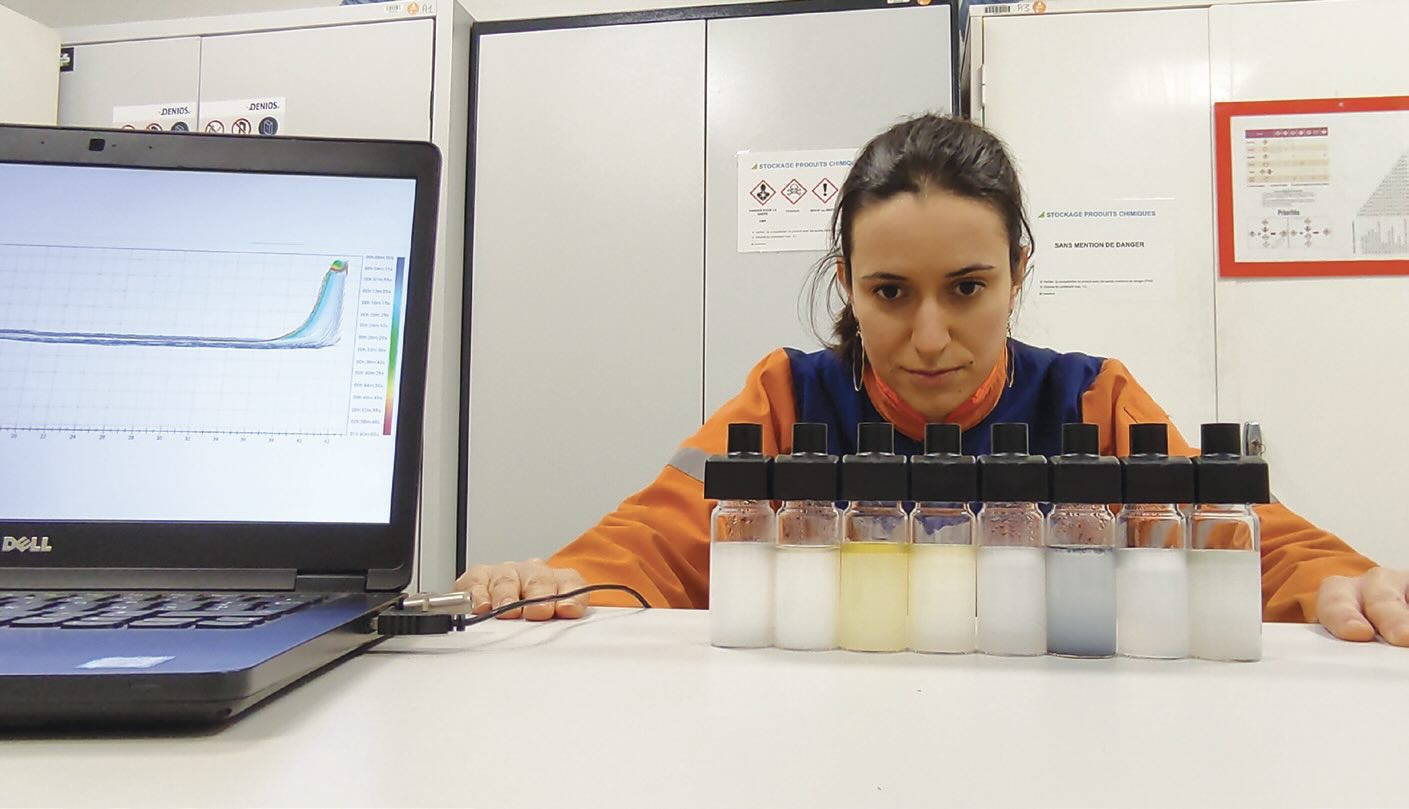 Ariane Viat evaluating different destabilization phenomena for various emulsions.
Ariane Viat evaluating different destabilization phenomena for various emulsions.
TLT: What are the main factors influencing emulsion stability?
Viat: Emulsion stability depends on three main factors: the chemical composition of the system (especially the surface-active properties of its molecules), the mixing process and the temperature.
Except for three particular element mixtures, the emulsion needs a surfactant that circles the droplets to stabilize them in the continuous phase. The surfactant molecule has affinity with both the continuous and dispersed phase, enabling a rather strong interface between them. For oil/water emulsions, the stability depends on the hydrophilic-lipophilic balance (HLB) of the surfactant that determines whether the surfactant prefers the oil or the water phase. When two surfactants with two different HLBs are added, bimodal PSD can be obtained. The smallest droplets basically give the most stable emulsions. The choice of surfactants depends on the nature of molecules to be stabilized, on the potential incompatibilities with some elements present in the emulsion (e.g., anionic-cationic compliance) and also on their toxicity toward aqueous media.
An emulsion also needs some mechanical stirring to be stabilized, because it helps to create smaller droplets. When comparing fresh emulsions in the lab, it is mandatory to ensure exact same stirring conditions for the different emulsions, other- wise the surfactant role cannot be decorrelated from the mechanical action. By the way, some surfactants result in stable emulsions after weak stirring, while some other surfactants reach similar stability only after high mechanical shearing.
As emulsions are thermodynamically driven, temperature plays an important role. The electrostatic forces maintaining the droplets in the dispersed phase can easily be destabilized by temperature changes. Most surfactants possess their surface-active properties only on a certain range of temperatures, typically between their pour point (solid-liquid transition phase) and their cloud point (solubility loss on the phase of affinity). Destabilizing an emulsion through high heating is a means used for further separation of waste emulsions.
One extra factor that was not mentioned among the main ones is the presence of solid particles (Pickering effect). Under particular conditions, some solid particles added to an emulsion can enhance stability. This is something I am studying in emulsions in use for aluminum rolling, as some worn particles from the aluminum plate contaminate the rolling emulsion. This work has been presented at the 2022 and 2023 STLE Annual Meetings.
TLT: How does the stability of emulsions for aluminum hot rolling influence the hot rolling process?
Viat: The rolling process needs an optimal emulsion stability to be efficient. Aluminum rolling is usually processed with oil-in-water emulsions with 1%-10% oil concentration range. The rolling process involves a sheet that is pushed (we say “bitten”) between two rotating rolls and is exited at reduced thickness. The rolls are sprayed with emulsion before the rollsheet contact (the “roll bite”), in order to lubricate and cool down the interface. At the entry of the roll bite, the “plate-out” phenomenon occurs; the oil and water separates. Only the oil enters the roll bite, whereas the water is rejected, mainly because water is incompressible and has poor affinity with the metallic surfaces in these conditions. The oil-water separation in the roll bite is necessary to ensure a continuous oil layer all along the sheet-roll contact, that prevents native aluminum to stick on the steel roll with subsequent risks of quality “pick-up” defects. To ensure sufficient plate-out, the emulsion needs to have sufficient instability!
On the other hand, emulsion also needs to have sufficient stability because it works in close circuit on the rolling mill. If the stability is insufficient, oil releases to the tank surfaces of the circuit and the emulsion loses its lubricious feature. In reality, even a sufficiently stable emulsion releases oil anyway, and to a certain extent, it is for the good; the oil skimmed at the emulsion surface contains many contaminants (tramp oil from hydraulic and mechanical circuits, aluminum worn particles).
In a nutshell, when emulsion stability is too high, there is a risk of discontinuous oil film in the roll bite, thus poor surface quality due to aluminum pick-up. High emulsion stability also means poor contaminant rejection, so insufficient emulsion cleaning. On the contrary, when emulsion stability is too low, oil concentration in the emulsion may be too low due to massive oil rejection. In this case, lubricity is poor and similar surface quality defects can occur.
The stability of an emulsion in use results in complex contributions of the surfactants, the way the emulsion is stirred through pumps and nozzles and the presence of contaminants (solid particles, tramp oils). For some emulsions, the lab version is always less stable than the industrial version, but for some others, the statement is reversed!
 Ariane Viat working in the lab.
Ariane Viat working in the lab.
TLT: What has been your most rewarding accomplishment throughout your career in the industry?
Viat: Industry is a very energy-intensive activity. I consider that as employees of an industry, we have a responsibility to contribute to reducing the carbon footprint of our business. We produce aluminum goods that will last for long hopefully, but that also have a finite lifetime, and use finite resources to be manufactured. In parallel, the lubricants used to produce aluminum are not fully renewable and even so, oily plants are grown through intensive agriculture. Something that I am proud of is having proposed an emulsion formulation with 50% reduced content in a non-renewable and rather harmful molecule. This formulation was tested on an industrial mill, which implies large industrial risk and complete emulsion change on a machine that has almost never seen any full dump. This emulsion formulation is still in place today. Even if this emulsion trial was not free from problems, I think we should collectively do things right in order to have such ambitious projects accepted by top management— and especially for projects that are rooted in the reality of our environment, not only of our company.
You can reach Ariane Viat at ariane.viat@constellium.com.