HIGHLIGHTS
•
Passivation of an aluminum metal surface initially leads to the formation of an aluminum hydroxide crystalline nature.
•
Introduction of more water vapor generates a bilayer oxide film with an amorphous aluminum oxide layer displacing the crystalline aluminum hydroxide near the metal surface.
•
The reason the inner oxide layer is amorphous is because this layer is thermodynamically favored, and the interface with the metal surface and the top aluminum hydroxide crystalline layer is energetically more favorable.
The growing use of aluminum in industrial and automotive applications is increasing as end-users take advantage of the metal’s light weight, which leads to improvements in efficiency. Aluminum forms a protective oxide coating that is stable up to pH values of 9.0. Above that pH, the coating dissipates leaving the metal surface vulnerable to corrosion.
But machining aluminum alloys with water dilutable metalworking fluids leads to staining as the metal can turn white, black and gray. Metalworking fluids are formulated to perform at a pH of 9.0 and do include additives known as metal deactivators that can protect against aluminum staining.
A past TLT article
1 discusses a study conducted to better understand how a specific aluminum alloy undergoes surface oxidation. The nickel-aluminum alloy examined has a stepped surface structure that contains a series of terraces or levels that are connected by single atom steps. Exposure to oxygen led to the formation of aluminum oxide stripes that grow on the terraces. Nickel atoms that are not vulnerable to oxidation diffuse into the bulk. As the oxide stripes continue to grow, the terraces bunch together eventually slowing and then stopping the oxidation process.
One of the authors of the past study, Guangwen Zhou, professor of mechanical engineering in the Thomas J. Watson College of Engineering and Applied Science at Binghamton University—State University of New York in Binghamton, N.Y., is working with colleagues to better understand how water interacts with aluminum at the atomic level to form a passivation layer. He says, “Surface chemical processes such as corrosion occur when water interacts with solids such as aluminum. Past studies have been limited in just demonstrating the reaction of water with small lengths of metal surfaces at cryogenic temperatures. But this approach does not provide insight on what occurs under technologically relevant operating conditions.”
The opportunity for evaluating how aluminum reacts with water under ambient conditions is now possible through the use of aberration-corrected environmental transmission electron microscopy (ETEM). Zhou says, “This technique allows for the very high-resolution analysis of gassurface reactions under ambient conditions. Transmission electron microscopy (TEM) is typically vacuum based. ETEM has the capability to differentially maintain a gas phase in the sample area. This allows for imaging of gas chemical reactions
in situ.”
Zhou and his colleagues studied the effect of water vapor on the surface of aluminum metal in an effort to better understand mechanistically how the oxide passivation layer forms.
Bilayer oxide film
The researchers worked with thin aluminum foils exhibiting a nominal thickness of approximately 50 nanometers. These materials are prepared using the focused ion beam lift-out technique and the NanoMill system. The foils were atomically cleaned through the use of a condensed electron beam inside the TEM to sputter off native oxide and generate well-defined facets. Deionized water is used in the study and is added to an aluminum foil through a leak valve at a given temperature and gas pressure.
Treatment of clean aluminum foils with various surface orientations and structures, such as [100], [111] and highly stepped surfaces with 3.5 x 10-5 torr of water vapor or liquid water, leads to the formation of oxide on the metal surface. Zhou says, “An initial ETEM observation identifies some kind of surface damage on the aluminum surface. Heat is generated as water is reacting with aluminum metal producing an exotherm. The result is the formation of a surface oxide build-up consisting of aluminum hydroxide as metal atoms are extracted from the topmost layer of the surface. This is the beginning of the formation of a passivation layer which initially is crystalline in nature.”
As water vapor continues to encounter the aluminum metal surface, a further transformation of the passivation layer occurs. Zhou says, “Additional water molecules attack deeper into the aluminum substrate eventually resulting in the formation of an amorphous aluminum oxide layer that displaces crystalline aluminum hydroxide. ETEM imaging shows that a crystalline aluminum hydroxide layer remains on top of the amorphous aluminum oxide layer which is adjacent to the metal surface.”
A TEM image showing the bilayer oxide film that acts as a passivation layer on aluminum is seen in Figure 1.
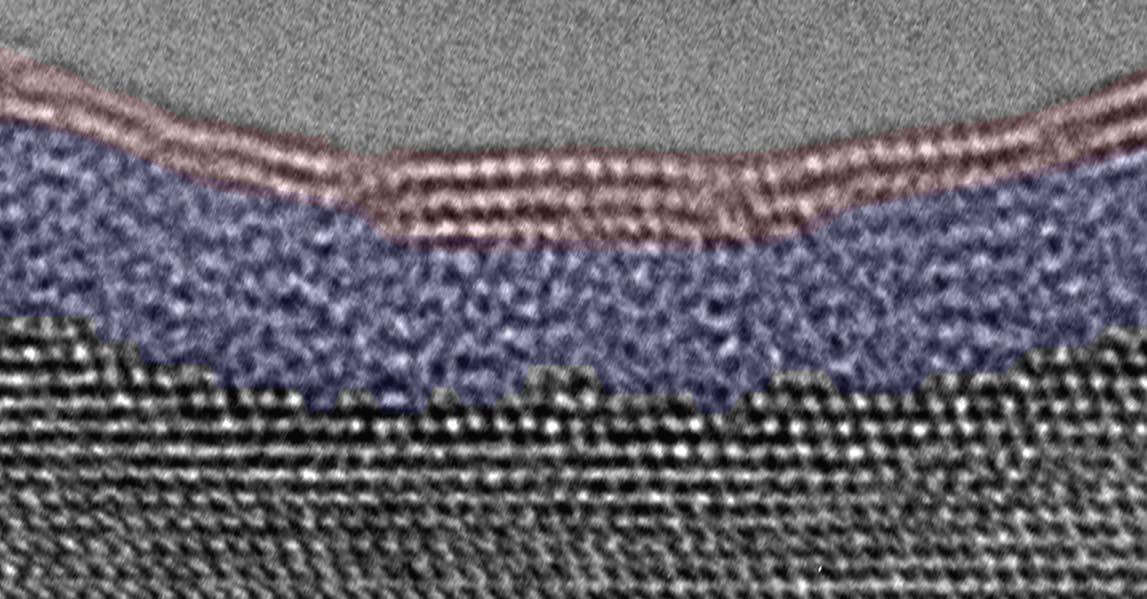 Figure 1. Passivation of an aluminum metal surface (grey layer) occurs with a bilayer oxide coating of amorphous aluminum oxide (blue layer), and crystalline aluminum hydroxide (red layer). Figure courtesy of Binghamton University—State University of New York at Binghamton.
Figure 1. Passivation of an aluminum metal surface (grey layer) occurs with a bilayer oxide coating of amorphous aluminum oxide (blue layer), and crystalline aluminum hydroxide (red layer). Figure courtesy of Binghamton University—State University of New York at Binghamton.
Chemical confirmation of the composition of the bilayers was carried out using ambient-pressure X-ray photoelectron spectroscopy (AP-XPS). One other issue AP-XPS was able to determine is what happens to the hydrogen atoms that are split from water during the reaction with aluminum. Zhou says, “Using AP-XPS and mass spectroscopy, we found that hydrogen gas is produced during the reaction with aluminum.”
Treatment of water vapor on an aluminum substrate that already has an amorphous aluminum oxide passivation layer produces a crystalline aluminum hydroxide layer. Zhou says, “This experiment proves that the bilayer oxide film is the most thermodynamically, stable passivation layer. Typically, crystalline phases are more stable than amorphous phases. However, the transition of the inner oxide layer to an amorphous phase is thermodynamically favored for several reasons, First, aluminum oxide is more stable than aluminum hydroxide. Additionally, this transition leads to energetically more favorable interfaces both with the aluminum substrate and with the top aluminum hydroxide layer.”
The outcome of this work will hopefully lead to the designing of materials that are more stable when exposed to the potential negative effects of water vapor. Zhou says, “We are going to evaluate how other metal alloys interact with water vapor on an atomic scale. Substrates to be evaluated include chrome steel and a nickel/aluminum alloy.”
One other phenomenon Zhou will consider evaluating is how the aluminum oxide passivation layer dissipates at pH values above 9. Information produced from this study may help in finding potential additives that can be used to minimize aluminum staining.
Additional information can be found in a recent article
2 or by contacting Zhou at
gzhou@binghamton.edu.
REFERENCES
1.
Canter, N. (2015), “Aluminum alloy acts to inhibit corrosion,” TLT,
71 (3), pp. 18-19. Available
here.
2.
Chen, X., Shan, W., Wu, D., Patel, S., Cai, N., Li, C., Ye, S., Liu, Z., Hwang, S., Zakharov, D., Boscoboinik, J., Wang, G. and Zhou, G. (2023), “Atomistic mechanisms of water vapor-induced surface passivation,”
Science Advances, 9 (44), DOI: 10.1126/sciadv.adh5565.