KEY CONCEPTS
•
Advanced fluid formulations can reduce foaming, but higher operating pressures and extended fluid life cycles often act to increase foam generation.
•
Metal fines, fatty acids, filtration, tankside treatments and other factors can increase or decrease foaming, depending on amounts and conditions.
•
Metalworking fluid customers and their suppliers must communicate to ensure the best match between fluid formulations and specific operating conditions.
Metalworking fluids (MWFs) prolong tool life by cooling and lubricating the areas where tools and workpieces come into contact. These fluids also wash metal chips and fines away from the work area to ensure clean, uniform surfaces on the workpieces. Excessive foam generation can interfere with each of these functions
(see Figure 1). Because foam bubbles are mostly air, they reduce the fluid’s ability to cool and lubricate, and foam can make it more difficult to remove metal particles from the MWF.
 Figure 1. Excessive MWF foaming causes a variety of problems in metalworking operations. Figure courtesy of Etna Products Inc.
Figure 1. Excessive MWF foaming causes a variety of problems in metalworking operations. Figure courtesy of Etna Products Inc.
Formulations have advanced to provide better performance, especially for the more fully synthetic fluids, says James Sullivan, global IFL technology director for additive manufacturer Münzing. On the other hand, customers are using fluids at higher pressures and extending the life cycle of their fluids, which increases the demand on the fluid. “Over time, the higher demand you put on that fluid, the more foam issues you get,” he says.
Fluid formulations
“The three components you need for foam are water, oil and air,” says John Fitzsimmons, director of technology for the MWF manufacturer Etna Products Inc. Fully or mostly synthetic MWFs, containing mostly water-soluble additives, present less of a problem than semisynthetic or emulsifiable oil formulations, which have oil, water components and an emulsifier to keep them stable and uniform. In those situations, he says, “You’ve got not only corrosion inhibitor additives but also lubricants and emulsifiers all working together.”
Formulators have access to a variety of lubricants for making water-dilutable MWFs, Fitzsimmons explains. Commodity and polymeric esters offer versatility in boundary lubrication performance for all metals, he adds. However, these esters can hydrolyze, forming fatty acids in a working coolant, which can cause foaming.
In some cases, however, a small amount of calcium soap particles (formed when hard water reacts with fatty acids) can retard foam formation. Thus, using too-soft water can cause foaming, explains STLE member Tom Oleksiak, senior technical manager with Quaker Houghton. He adds that his previous experience with formulating and designing low-foam emulsifiers has taught him that you can’t use certain additives in semisynthetic fluids. Additives that can cause foaming problems include high-foaming surfactants and even certain antioxidants and corrosion inhibitors.
Generally, the longer the hydrophobic tail of a surfactant molecule, the less likely it is to generate foam, he says.
1 Multiple hydrophobic tails on a molecule also help in minimizing foam. In his experience, the formulations most likely to generate foam involve semisynthetics with high levels of emulsifiers. He has observed the fewest problems with soluble oils and low levels of emulsifiers, but he adds that well-formulated synthetics also rarely suffer from foam problems.
Ethylene oxide or propylene oxide polymers added to synthetics produce an inverse solubility effect (the warmer the fluid, the less soluble these polymers are) that destabilizes foam, Oleksiak explains. You can add propylene oxide or cap the surfactant with propylene oxide to lessen foam
(see Figure 2a). These simple organic species are readily available at a reasonable price. “The propylene cap is far and away the most common option,” he says.
A less-common option is gemini surfactants, two molecules with hydrophilic heads and hydrophobic tails linked with a spacer to form a dimer
(see Figure 2b). Because each dimer molecule can anchor within one oil molecule, less surfactant can be used, which reduces the amount of foam.
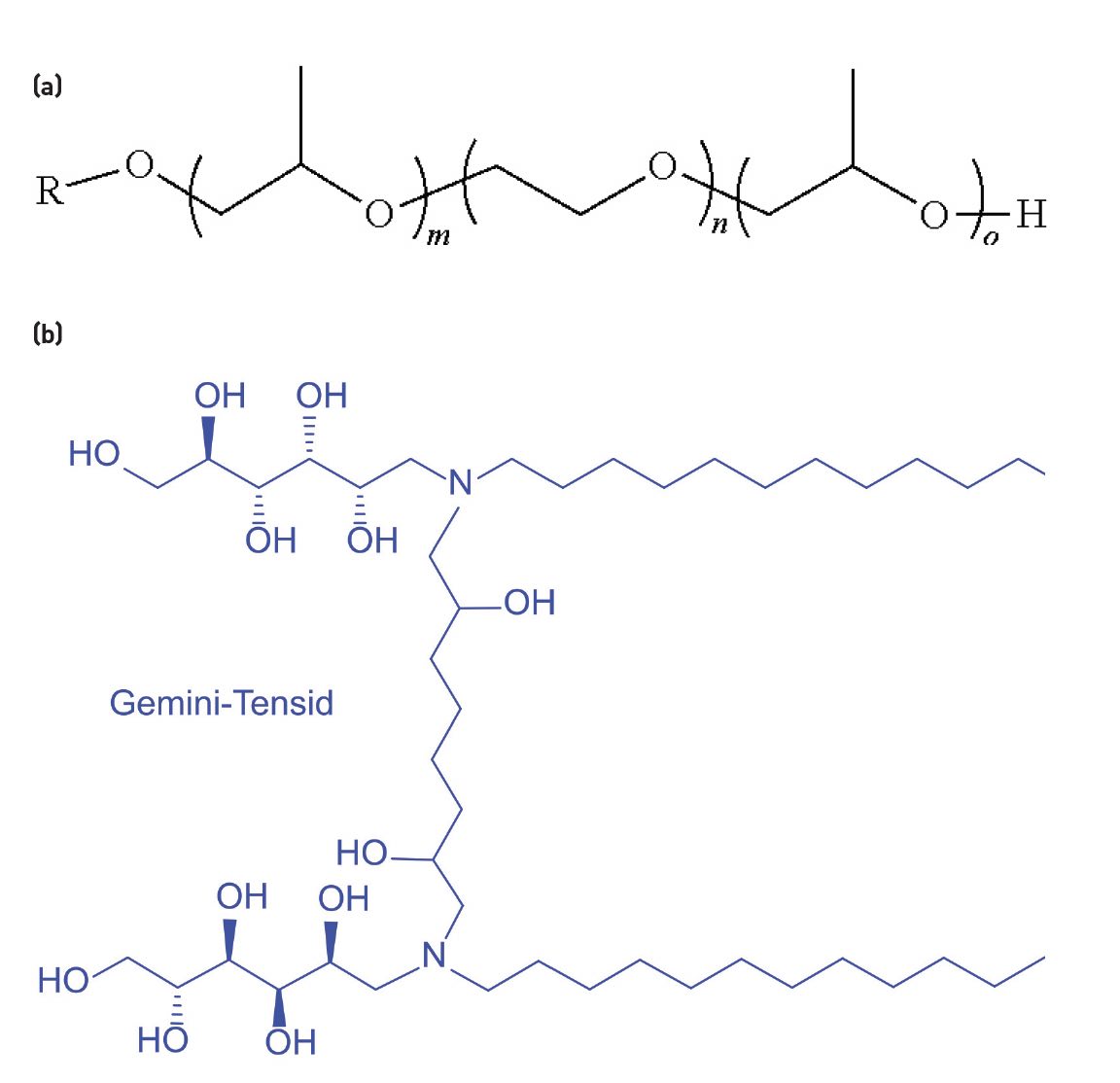 Figure 2. (a) Propylene oxide-capped polymeric surfactant molecule. (b) Gemini surfactant molecule. Figure 2a courtesy of U.S. Patent US20220095613A1, public domain. Figure 2b courtesy of ChemDoc 2010/Wikimedia Commons, CC BY-SA 4.0.
Figure 2. (a) Propylene oxide-capped polymeric surfactant molecule. (b) Gemini surfactant molecule. Figure 2a courtesy of U.S. Patent US20220095613A1, public domain. Figure 2b courtesy of ChemDoc 2010/Wikimedia Commons, CC BY-SA 4.0.
“I think all metalworking chemists would like to have there be a generalized solution to foam,” says Oleksiak, “something that you could put in your product and it’s readily soluble, it doesn’t get filtered out and it always kills foam. If that’s been developed, I’m not aware of it.
“A defoamer works at the interface, and it needs to stay at the interface, so it’s not especially soluble in anything,” says Oleksiak. However, it does need to be somewhat miscible with the MWF so that it doesn’t separate out during storage. “It doesn’t matter which phase it’s soluble in as long as it stays somewhere in the fluid and doesn’t filter out during use.” Customers often buy and store MWFs in large drums or 400-gallon totes. Although retail consumers are accustomed to seeing “shake before using” instructions on small containers of salad dressing, paint or other products, he adds, “there’s no tank truck shaker” for bulk quantities of MWFs. “Even if it’s only 0.1% of a 5,000-gallon bulk order, that’s 5 gallons of goo sitting on the bottom of the tank truck.”
“You’re always trying to balance compatibility with defoamer performance because if a defoamer is fully soluble in a fluid, it’s not going to perform,” Sullivan says
(see Figure 3). On the other hand, he adds, if the defoamer is not soluble enough, it separates out of the fluid and becomes ineffective. His lab rates defoamers based on their presence at a fluid’s surface, body (hazy appearance) and bottom, to show where kickout is occurring.
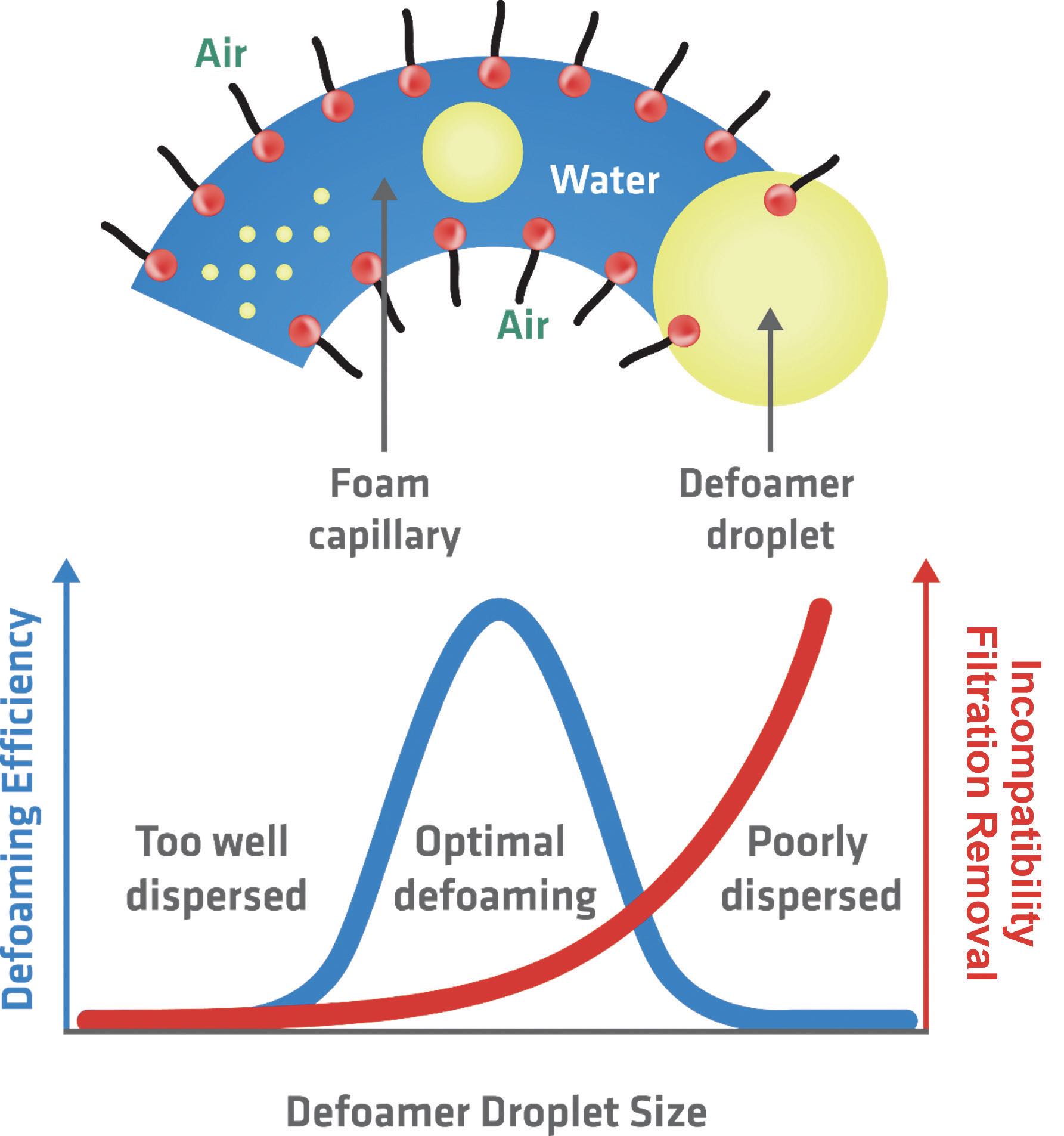 Figure 3. Defoamers (yellow) work most effectively when they are moderately well dispersed within a water-containing MWF (blue), forming optimally sized droplets within the fluid. (Red circles with black tails represent the organic components of the MWF.) Figure courtesy of Münzing.
Figure 3. Defoamers (yellow) work most effectively when they are moderately well dispersed within a water-containing MWF (blue), forming optimally sized droplets within the fluid. (Red circles with black tails represent the organic components of the MWF.) Figure courtesy of Münzing.
In an ideal world, you could formulate a different fluid for every customer, but that’s just not realistic, says STLE member Josh Miller, senior development chemist at QualiChem. Formulations for different geographic regions, applications or water types are a much more achievable goal. Water quality, especially hardness, is an important factor for soluble oil concentrates (which must be diluted with water before use) and semisynthetic fluids, because of the need for emulsifiers that keep the oil and water phases mixed. Straight oils are less likely to foam, Miller says, adding that fully synthetic fluids can foam, but most foaming issues come from semisynthetics and soluble oils.
MWFs can become depleted in specific additives (for example, long-chain fatty acids or low cloud-point surfactants) as they age, says Oleksiak, and very fine filters can strain out defoamer molecules. Tankside treatments can help by replenishing additives, including polysiloxane defoamers and low-foam surfactants. Silicone-based defoaming agents contain emulsifiers and couplers and are intended to release a thin film at the liquid-air interface in systems that prevent the formation of bubbles, Fitzsimmons says. Over-treating the system can allow the defoamers to build up into a thicker film that is more easily removed during filtration or carried away by tramp oil or skimmers, leaving only the emulsifiers and couplers from the defoamer and adding to an already foamy system.
Fitzsimmons notes that he has not seen drastic changes in customer demand for MWF formulations. Mostly, he says, they want a fluid that works for their applications and processes, especially if these involve high fluid delivery rates or high pressures. “Foam is always a consideration,” he says. However, a push toward greener products is on the horizon, probably starting with end-use customers and tier one parts suppliers, especially in the automotive sector, and then making its way to fluid manufacturers and similar suppliers. The push also could come from feedstock suppliers, he adds, driven by supply chain changes and regulations affecting specific components.
Miller agrees—one major driver in the ongoing work of formulation development is a push for greener technologies. However, complexity enters into this effort as well. Formulators are challenged to create fluids that perform well, use more sustainable raw materials, produce less pollution—and do it all for a reasonable price.
Sullivan has noticed an increasing demand on both the customer side and the regulatory side for more sustainable MWFs, including fluids based on biodegradable, renewable or biobased materials. These products must incorporate components that draw on environmentally responsible feedstocks, and they must not cause pollution or adverse health effects. In addition, the components must be compatible with each other and contribute to effective performance. Sustainable MWFs must do all this, while performing under high pressure for long periods of time, and they must be economical to buy and maintain, he adds.
Tankside defoaming treatments, provided as a water-based emulsion with a biocide, also can be a concern, he adds. Highly concentrated products are less convenient to handle, and they can result in overdosing, which impedes dispersion in the tank and can cause components to separate out. “For tankside applications, usually your best delivery mechanism is in a water-based carrier, as an emulsion.” These emulsions typically include biocides, thickeners and other ingredients, he adds, and these ingredients must all conform to health and safety standards.
Operating conditions
Application pressure and the system size have the most influence on foam generation, Oleksiak says. High-pressure applications offer more efficient cooling and can prolong tool life, but they are the most prone to generating foam or entrained air. This air has a chance to come out of the fluid if it has a long enough residence time in the sump. However, with a move toward smaller sumps, foam and entrained air have less time to dissipate before the fluid is put back into service.
Miller notes that many customers are pushing for faster production, which can cause foam by reducing the time for the fluid to release entrained air. High-pressure machine types, operating above 800 psi, also can contribute to foam. Miller advises his customers to evaluate what throughput rates are necessary for their operations and not just drive for faster speeds regardless.
Users need to distinguish between foam, which forms on the fluid’s surface, and entrained air, which is dispersed throughout the fluid, Oleksiak says. “We’re better at testing foam than entrained air,” he adds. “We’re trying to sort out which products work well for low foaming and which products and surfactants minimize air entrainment. They tend to be related, but not necessarily directly related.” Just because a product is low foam doesn’t mean that it’s not going to entrain air, he explains. Also, entrained air can rise to the surface and become foam. “As we’re going to higher-pressure systems, we need to understand more about air entrainment, but that’s still something we’re gaining knowledge on.”
Filters can affect foam formation, Sullivan says. His lab has a standard defoamer test method that they use for aqueous MWFs. He describes it as a scaled-down version of the CNOMO recirculation test
(see Figure 4), which uses a peristaltic pump to circulate the fluid through a graduated cylinder at a specified pressure for a specified period of time.
2 “We have found that to be very good at simulating what happens in the field in terms of which defoamer is going to perform the best,” he says. They also can install an inline filter to evaluate how the defoamer works with the filter’s chemistry and pore size. This test is currently being evaluated by ASTM for recognition as a standard practice.
 Figure 4. Recirculation test apparatus for evaluating defoamer performance. Figure courtesy of Münzing.
Figure 4. Recirculation test apparatus for evaluating defoamer performance. Figure courtesy of Münzing.
Filter pore size can contribute to foaming in several ways. Very fine filters can screen out additives, including defoaming agents, for example. However, filter chemistry is often more important than pore size for maintaining defoamer performance, Sullivan says. Because defoamers are hydrophobic compounds, they are attracted to hydrophobic filter materials like polypropylene, more so than more polar filter materials like nylon, especially when the fluid itself is water-based.
3,4 Sullivan notes that because defoamers are less likely to be adsorbed onto a more polar filter material, customers can use a nylon filter with a smaller pore size and catch more fine particulates without taking the defoamer out of the fluid.
Fitzsimmons says that some of the newer machines have their own inline screens that must be cleaned regularly to prevent metal debris from clogging them
(see Figure 5). As with other filters, debris buildup can restrict fluid flow and contribute to foam formation, he explains, but their less-visible inline location can make them easy to forget. Bag filters also can present a problem, he adds, because debris buildup in these housings is more difficult to monitor. Thus, routine inspection and maintenance of these less-visible filters (as well as the more visible ones) is critical to foam prevention.
 Figure 5. Routine inspection and maintenance of filters helps prevent foaming caused by an accumulation of metal particles and other debris. Figure courtesy of Etna Products Inc.
Figure 5. Routine inspection and maintenance of filters helps prevent foaming caused by an accumulation of metal particles and other debris. Figure courtesy of Etna Products Inc.
Contamination
Contaminants in the MWF also can contribute to foaming. Even synthetic fluids can produce foam if they become contaminated with tramp oil, metal fines or residual cleaning products.
Depending on the conditions, metal fines in the MWF can be a help or a hindrance. “If the metal fines are small and light enough to float in the fluid, they can get to the liquid- air interface and physically pop the bubbles,” Fitzsimmons says. However, if the chips are large enough and heavy enough to restrict fluid flow, then they can add to foaming problems.
Oleksiak works mainly in the area of aluminum rolling oils, which do not experience as many foaming issues as other operations. In part, this is due to the nature of rolling, rather than, say, machining or broaching, but the small fine particles (less than 10 micrometers across) generated during rolling can help in breaking up any foam that might form, he says. However, even here, foaming can be a problem during startup, before a sufficient amount of fines enters the fluid.
Although metal fines can help break up surface bubbles, Sullivan explains, if the fluid does not have a good defoamer, or the defoamer concentration is too low, metal fines can get caught up in the foam and form a crusty surface, “like you see on top of a river.” He adds that this crust can be very hard to get rid of. “You need a good defoamer to prevent them from getting caught up in the bubbles. Then, they can settle to the bottom so that they can be filtered out.”
Accumulations of metal chips can restrict flow and increase pressure in the delivery lines, Fitzsimmons explains. If the system has a recovery area like a sump, where the fluid can move outside the turbulent zone, then the antifoaming agent in the fluid can destabilize and dissipate the foam, allowing the fluid to circulate through the system properly.
Oil contamination is another potential culprit in MWF foam formation, Fitzsimmons says. Maintenance oils, hydraulic oils or gear oils can work their way into the MWF stream. However, he adds, a good MWF formulation will either emulsify or reject those oils so that they do not compromise the performance of the MWF.
Miller has seen situations where tanks and delivery systems have not been rinsed thoroughly after cleaning. Many cleaning products are purposely made to produce large amounts of foam, he says. Residual cleaner can mix with the MWF and cause a foaming problem.
Testing methods
In the past decade, predictive test protocols have been developed for foam formation in water-miscible MWFs, but as recently as 2021, there was no single protocol that was universally suitable for this purpose. Not only that, but there are no generally recognized standard MWF or foam control additive fluids, so reference fluids must be relevant to the type of test being performed.
5
Although the recirculation test is a standard evaluation method for defoamer performance, Sullivan notes that various laboratories also have come up with innovative ways to simulate specific problems in the field,6 including such setups as a fish tank with a waterfall to simulate the effects of turbulence and splashing. They also run their tests at various temperatures, using heat-jacketed cylinders.
Much testing involves comparing a fluid manufacturer’s formulation with and without additives, but without a specific knowledge of the other components, because of the manufacturer’s intellectual property concerns. That, and the system-specific factors of the end-user’s applications, places limitations on what can be tested. However, many companies keep thorough data on the products made and the results of tests, which are included in product recommendations.
MWF formulators test various filters, sump sizes, storage temperatures, application pressures and temperatures and contact zone temperatures as they develop new fluids, and they can recommend operating parameter ranges to their customers. Storage temperature is not as much of an issue with defoamer stability as for some other additives. “If it’s going to come out, it’s going to come out at all temperatures,” Oleksiak explains. However, formulators do test their fluids over a range of temperatures to check their overall performance, he adds.
Fitzsimmons notes that MWF formulators put their products through a variety of tests to ensure that they do not produce excessive foam under high fluid shear and high-pressure spray conditions. Typical formulations work well up to about 500 or 600 psi, he says, but special low-foaming formulations may be required for higher pressures, in the 800 to 2,000 psi range.
He explains that sometimes unforeseen problems arise when new machines are put into service. If the customer runs a benchmark product with all the spray ports open, everything may appear to be in order. However, if they turn off some of the spray ports or otherwise modify the spray environment during normal operations, the higher pressure at the open ports can result in foam formation. Likewise, changing over to a different brand of fluid or a different formulation may require further tuning of the flow rates and pressures to get the best performance. Fitzsimmons notes that many customers have worked with their machines long enough to be well acquainted with their features and requirements, and OEM representatives typically work with customers who are setting up a new machine.

In the future, Fitzsimmons would like to see modifications to the commonly used CNOMO recirculating pump test
2 that would enable it to be used at flow rates and pressures more typical of today’s operations. It also would be useful to be able to change out nozzles on the test rig, he adds. The current test rigs have the benefit of being small-scale and lab-based, but sacrificing some of that convenience might be worth it to be able to replicate something closer to real-world operations. Some manufacturers have developed their own larger-scale tests, which benefit from more realistic conditions but also generate more fluid waste. “If they could come up with a more efficient way of testing high-pressure foam on a lab bench scale and use less than a gallon of coolant, that would help the industry tremendously,” he says.
Communication is crucial
Miller notes that controlling foam is a complex issue in general because of the many factors that contribute to foaming. He visits customer sites whenever he can—“That’s where you learn the most, from talking with your customers and seeing what they are going through, and what their situation is,” he says. “Every customer is different.” The issue can be with the fluid or the water quality, or it can be as simple as a nozzle not pointing in the right direction, he adds. Most of Miller’s customers work with cutting and grinding applications. In general, metal types can affect the choice of an MWF, and this includes the metal that the machine and tool tip are made from, as well as the workpiece.
The fluid formulation has to be right for the specific application, Miller says. He notes that fluid formulators often hold many of the specifics on chemical interactions and antifoaming formulations very closely as proprietary information. Thus, good communication between the MWF manufacturer and the end-user is essential, rather than relying on the customer to figure things out for themselves. “You have to make sure that your customer knows exactly what they need in their application,” Miller says. “You have to have all the information, because if you only have part of the information, that’s really where you run into trouble. It’s usually what you don’t see.”
Artificial intelligence might eventually help with developing new solutions, but currently it’s better at finding patterns in existing information than for predicting new technologies, Miller says. “It’s just an extra tool,” he adds. For the most part, he says, he uses data and feedback from customers who provide information on water hardness, machine specifications and age and fluid concentrations. He notes that he gets detailed feedback from customers and takes the time to work through potential issues in detail.

One thing I’ve learned is no two shops are the same,” Miller says. He has seen significant differences in water quality between adjacent counties. Some shops use deionized water, while their neighbors use tap water, or they use the same machines but have different sump sizes.
Technical services laboratories work with end-use customers to help solve problems with specific applications and machine setups. Sullivan notes that today’s fluids are generally effective, so tool failure because of foam is not common. A more common situation, he says, is where a customer wants to extend their average tool life even further by using a more advanced product with less foaming.
Labs also do water testing, Sullivan says. Deionized water typically produces the most foam, whereas hard water can affect the stability of the fluid components. “If a customer knows their water hardness, we will make up that water hardness [in the lab] and do testing with that. That’s always something that we ask.”
Fitzsimmons notes that whether customers are using a well-established MWF or something newer, if a foaming problem arises, they generally call their supplier’s sales or customer support representative before a problem results in tool failure. Typically, the support rep begins with a mechanical investigation to see if the machine or something in the process is behind the foam generation. If it’s not a mechanical problem, the chemists from the technical group are brought in to see if the fluid in use is behind the issue.
“We ask questions like, has there been any kind of change in setup or the process?” Fitzsimmons says. “Is there a new machine installed versus a grandfathered machine that has been working for a number of weeks, months or years? Is there a new operator, and has the new person been trained on the machine or the process of whatever part they’re making? Have they changed machine settings? Have they added spray nozzles, or washdown feeds or anything to increase fluid delivery to the worksite? If there’s too much fluid to the worksite, does it have the capability of leaving the worksite at an acceptable exchange rate? Because if not, the increased volume of fluid will result in increased fluid turbulence that could add to foam in those situations.”
He notes that if a customer is considering a new fluid, they should compare its performance and characteristics to the fluid already in use for a given process. A supplier’s technical support representative can help arrange for testing and evaluation to help with these comparisons before the new fluid is put into service, not only to avoid potential problems but also to provide data on whether and how much the new fluid improves the process.
Regardless of whether foam is coming from a mechanical issue, a problem with the fluid formulation or maintenance or some other source, the customer’s main concern is keeping foam down to a level that lets them extend their tool life, produce high-quality parts, minimize waste and expenses and maintain a safe, healthy work environment. “Any kind of water-dilutable MWF can produce some amount of foam in undesirable conditions,” Fitzsimmons says. “Identifying those conditions and offering solutions and better products to help correct them is our job. So, foam control is always going to be a hot topic.”
REFERENCES
1.
Oleksiak, T., Ineman, J., Schultz, J., Ford, M. and Hughes, K. (2014), “Emulsifiers 201: Analyzing performance with surface science,” TLT,
70 (9), pp. 24-33. Available
here.
2.
Canter, N. (2015), “Foam: Dealing with a persistent problem,” TLT,
71 (12), pp. 24-38. Available
here.
3.
Sullivan, J. (2021), “Effects of Filtration on MÜNZING’s Next Generation FOAM BAN® Defoamer Technology in Aqueous Metalworking Fluids,” TLT,
77 (11), pp. 62-64. Available
here.
4.
Galgoci, E., Panzariello, J., Sullivan, J. and Mykietyn, J. “Effects of Filtration on Defoamer Performance in Aqueous Metal Removal Fluids,” 2017 STLE Annual Meeting & Exhibition, Atlanta, Ga.
5.
ASTM E3265-21: Standard Guide for Evaluating Water-Miscible Metalworking Fluid Foaming Tendency. Available
here.
6.
Mykietyn, J., Sullivan, J., Galgoci, E. and Panzariello, J. “Critical Examination of Foam Test Methods for Aqueous Metal Removal Fluids,” 2017 STLE Annual Meeting & Exhibition, Atlanta, Ga.
Nancy McGuire is a freelance writer based in Albuquerque, N.M. You can contact her at nmcguire@wordchemist.com.