Reducing friction in reducing environments
By Drs. Wilfred T. Tysoe & Nicholas D. Spencer, Contributing Editors | TLT Cutting Edge February 2024
Removing oxygen from the environment enhances the formation of lubricious carbonaceous films on metal substrates.
It has been known for about 70 years that hydrocarbons can react directly at sliding metal interfaces, depositing carbonaceous films that can lower friction. They are often referred to as friction polymers. This raises the question as to whether they might provide a viable approach to lubricating sliding interfaces. Indeed, STLE Past President Ali Erdemir, now at Texas A&M University, has exploited this general idea by coating steel surfaces with reactive metals to promote the formation of lubricious carbonaceous films by reacting with a poly α-olefin lubricant.
However, it turns out that the tendency to form carbonaceous films is quite variable and appears to depend on the atmosphere, especially whether it contains oxygen, and the nature of the surface. Substrates that contain reactive metals such as molybdenum, copper, platinum or palladium tend to more easily form carbonaceous films, while such films form less easily on steels.
STLE members professors Janet Wong and Hugh Spikes, with research associate Jie Zhang and student Bastein Bolle from Imperial College, London, investigated this problem in greater detail using environmental tribometers with enclosed sliding or sliding/rolling ball-on-flat configurations. In one tribometer, the enclosure volume was small enough to allow the gas composition to be rapidly changed to gauge the reaction kinetics. The sample was analyzed either
in situ or
ex situ by Raman spectroscopy to monitor how the surface composition evolves.
Raman spectroscopy is ideally suited for this purpose because the measured signals due to molecular vibrations are sensitive to both carbon and surface oxides, but not to the metal substrate. In addition, the laser beam that stimulates the Raman scattering can be focused to compare the products formed within the wear track with those found outside it.
Figure 1a shows the influence of nitrogen and dry air atmospheres on a hexadecane lubricant. The friction signal in dry air is large and erratic, while that in an inert nitrogen atmosphere is much lower (~0.2) and very stable. The Raman spectra below compare the surface composition in dry air
(see Figure 1b) and nitrogen
(see Figure 1c). The surface rubbed in air comprises almost exclusively an iron oxide film, while that in dry nitrogen exhibits the so-called D and G vibrational bands characteristic of carbonaceous films. However, it is also found that the film is easily removed, because the friction coefficient rapidly rises to almost unity once the hydrocarbon feed is turned off.
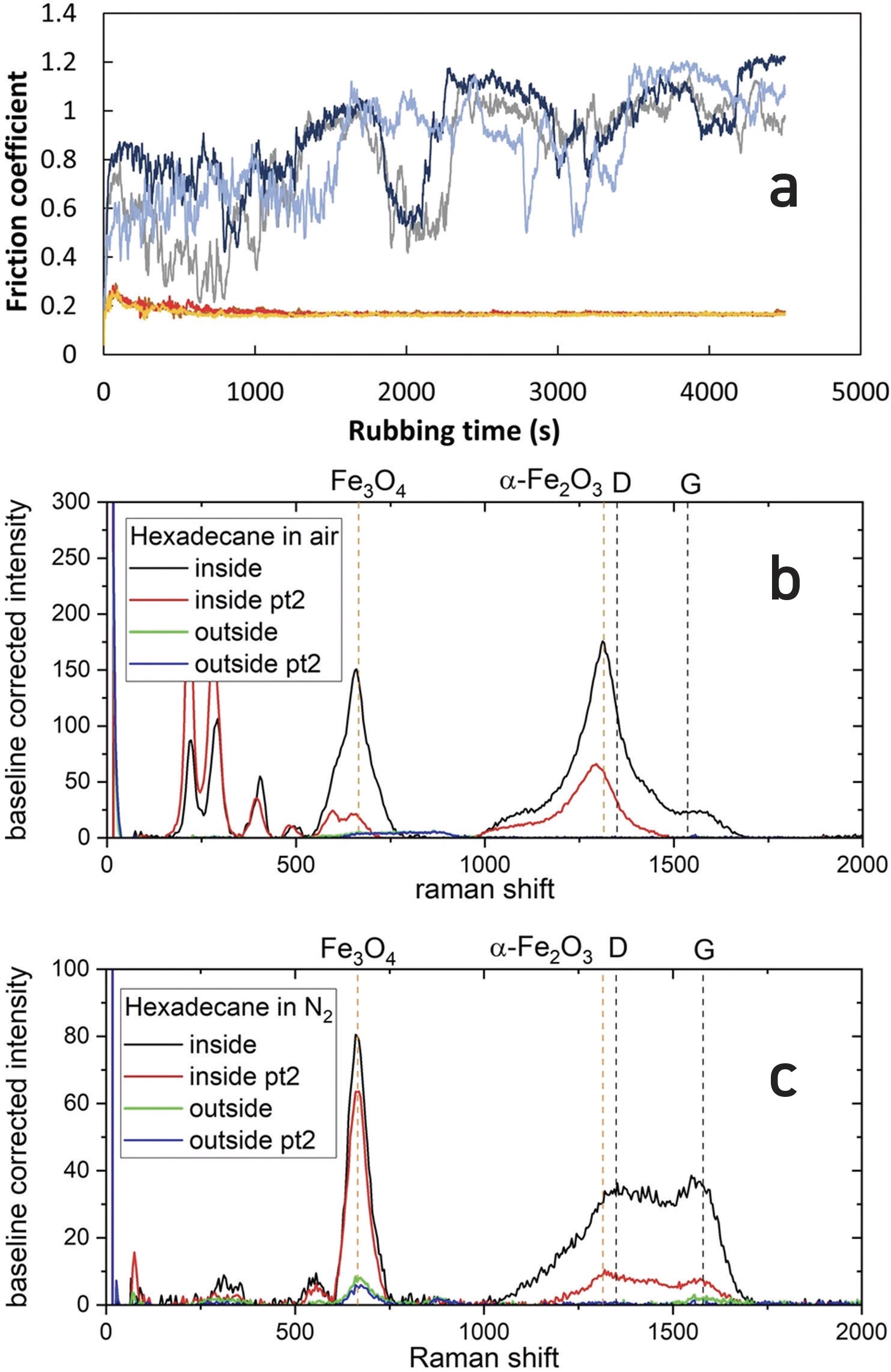 Figure 1. (a) Friction traces for hexadecane in dry air and in N2. Noisy blue/grey traces are in dry air, lower smooth red/orange traces are in N2. Three repeats are shown for each atmosphere. Test conditions, 1.92 N load, 60 °C. (b) Raman spectra of steel wear tracks formed in hexadecane in dry air and (c) in N2. Published with permission from Ref. 1.
Figure 1. (a) Friction traces for hexadecane in dry air and in N2. Noisy blue/grey traces are in dry air, lower smooth red/orange traces are in N2. Three repeats are shown for each atmosphere. Test conditions, 1.92 N load, 60 °C. (b) Raman spectra of steel wear tracks formed in hexadecane in dry air and (c) in N2. Published with permission from Ref. 1.
The response to a change in the atmosphere was measured by monitoring the friction coefficient after rapidly changing the gas composition from dry air to nitrogen, and the results are displayed in Figure 2. This shows the results of three distinct experiments carried out under identical conditions. While there are differences between experiments, the trends are clear. While the gas composition changes almost instantaneously, the friction responds more slowly. A reproducible trend emerges by the third cycle, during which the friction decreases rapidly after about 20 s of rubbing in nitrogen, presumably due to the formation of a carbonaceous film covering the surface. The switch to dry air induces a slower friction rise over a period of ~2.5 min, reflecting the carbonaceous-film removal kinetics.
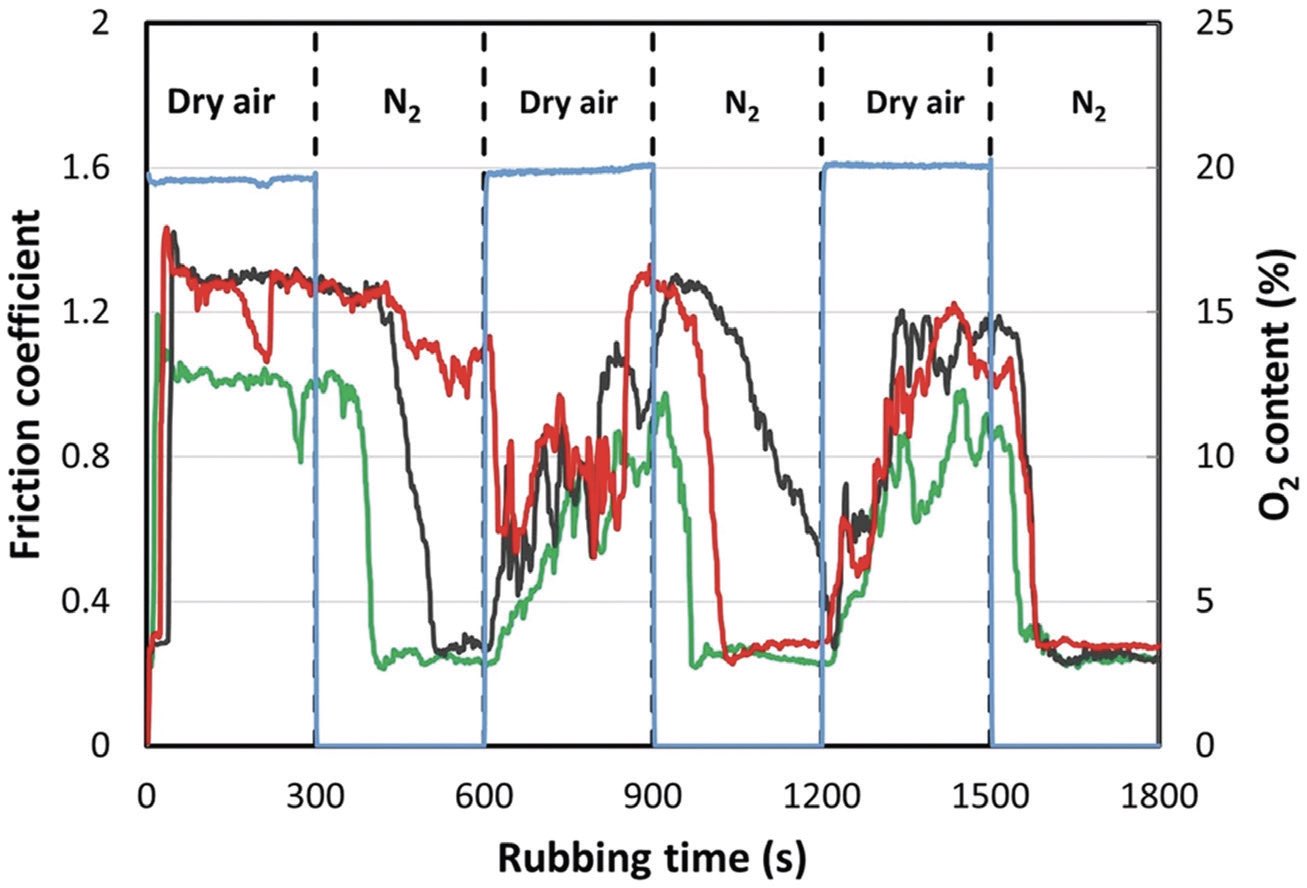 Figure 2. (a) Variation of friction coefficient of isooctane at 25 °C when switching between a dry air and a nitrogen atmosphere. Blue line shows variation of O2 concentration during the experiments. Published with permission from Ref. 1.
Figure 2. (a) Variation of friction coefficient of isooctane at 25 °C when switching between a dry air and a nitrogen atmosphere. Blue line shows variation of O2 concentration during the experiments. Published with permission from Ref. 1.
These results clearly show the potential of carbonaceous films formed in oxygen-free environments as viable lubricants. It is therefore appropriate to ask how they compare with more conventional lubricant additives. The answer is given by the results displayed in Figure 3, which compares the friction of a hydrocarbon in nitrogen with common friction-modifier additives, GMO and MoDTC in dry air.
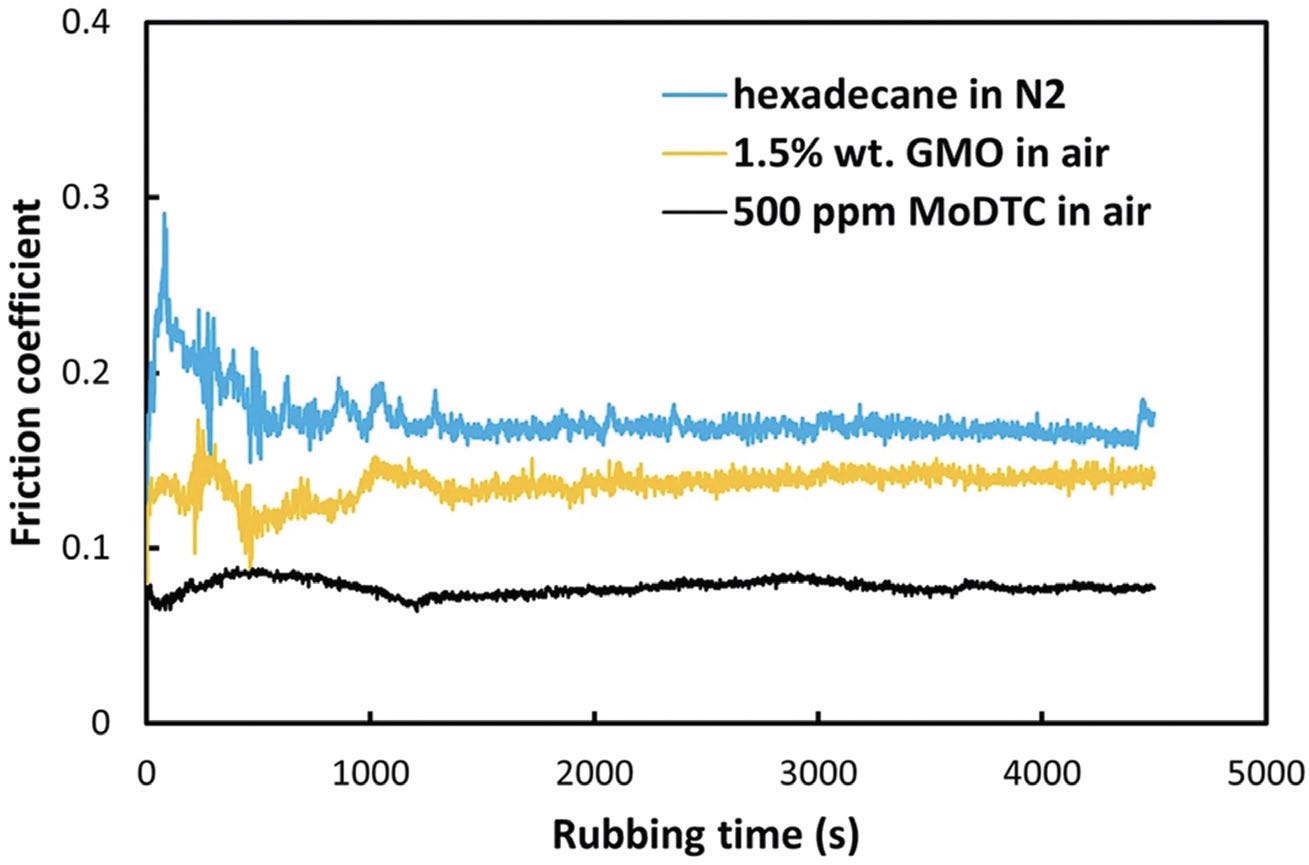 Figure 3. Friction traces of hexadecane in N2, glyceryl monooleate (GMO) and molybdenum dialkyldithiocarbamate (MoDTC) in hexadecane in dry air (60 °C). Published with permission from Ref. 1.
Figure 3. Friction traces of hexadecane in N2, glyceryl monooleate (GMO) and molybdenum dialkyldithiocarbamate (MoDTC) in hexadecane in dry air (60 °C). Published with permission from Ref. 1.
It is clear that the best friction modifiers that have been developed over several years perform better than a carbonaceous film grown from hexadecane. Of course, different hydrocarbon structures, possibly those with unsaturated bonds that increase the reactivity, may perform better. Hydrocarbons also may find applications in cases where common additives cannot be used or in synergy with formulated lubricants to extend their operational range, or perhaps even improve their properties. However, clearly, whatever the application, it should involve a low or zero oxygen environment.
REFERENCE
1.
Zhang, J., Bolle, B., Wong, J.S.S. and Spikes, H.A. (2024), “Influence of atmosphere on carbonaceous film formation in rubbing, metallic contacts,”
Tribology Letters, 72, 4.
Eddy Tysoe is a distinguished professor of physical chemistry at the University of Wisconsin-Milwaukee. You can reach him at wtt@uwm.edu.
Nic Spencer is emeritus professor of surface science and technology at the ETH Zurich, Switzerland, and editor-in-chief of STLE-affiliated Tribology Letters journal. You can reach him at nspencer@ethz.ch.