•
A non-aqueous redox flow battery was prepared without a membrane and with anolyte and catholyte electrolytes that are not compatible with each other.
•
The battery demonstrated a capacity retention rate of 81% over 100 cycles that took 45 days to complete.
•
No evidence of material degradation or solvent crossover was found during battery operation.
Research is ongoing to develop and commercialize electrical energy storage systems that can be used to complement renewable energy sources such as wind and solar. The objective is to have energy storage systems that exhibit excellent efficiency and long-term durability.
Initial work has been done with aqueous redox flow batteries that contain two massive electrolyte solutions (anolyte and catholyte) separated by an ion-exchange membrane. The anolyte is situated near the anode of the battery and contains materials that are reduced during battery charging. In contrast, the catholyte is situated near the cathode of the battery where materials are oxidized during battery charging. The ion-exchange membrane is designed to prevent crossover between the catholyte and anolyte during battery operations to minimize self-discharging.
In a past TLT article,
1 researchers developed a new catholyte based on sodium and potassium salts of iron (II) complexes of bipyridine dicarboxylic acid and dicyanide. This catholyte demonstrated superior stability and water solubility. Since the catholyte contains bulky molecules, the charged species produced during battery operation are readily blocked by the membrane and cannot crossover to the anolyte. The researchers achieved a capacity utilization of 85% with the battery maintaining 90.5% of its initial value after 6,000 cycles using this newly developed catholyte.
Dr. Rajeev Gautam, postdoctoral researcher at the University of Cincinnati in Cincinnati, Ohio, says, “While aqueous redox flow batteries exhibit positive features such as fast reaction kinetics, low cost, nonflammability and high ionic conductivity, they are limited by only being able to provide an operating voltage of 1.23 V and also display a limited energy density between 20 and 50 watt-hours per liter. The membrane needed represents a significant expense that is 30%-40% of the total cost of the battery.”
Gautam points out that preparing a non-aqueous redox flow battery should provide higher potential operating voltages (up to 6 V) and higher energy densities. He says, “Finding membranes that are effective in minimizing crossover between the anolyte and the catholyte has proven to be difficult. The problem is that most organic-based electrolytes are able to dissolve the polymer membrane and also are miscible with each other.”
One option is to design a membrane-free non-aqueous redox flow battery that possibly could reduce the cost. Gautam says, “The difficulty in identifying non-aqueous solvent systems is to find the right viscosity, and make sure the anolyte and catholyte do not mix with each other. They need to have different solubility properties to render them immiscible when exposed to each other.”
Past work conducted with membrane- free non-aqueous redox flow batteries showed promise but could not be scaled up from the microscale making them unsuitable for use as large energy storage systems. Gautam and his colleagues have now designed a non-aqueous redox flow battery without a membrane that shows potential for high-energy storage.
Lithium-based
The researchers developed a non-aqueous flow battery with anolyte and catholyte electrolytes that are not compatible with each other. Gautam says, “We worked with an ionic liquid based on a pyrrolidinium sulfonyl imide as the anolyte and a series of catholytes including triazine, phenothiazine and cyclopropenium derivatives mixed with fluoroethylene carbonate as the catholyte. The battery is lithium-based with the metal being used as the anode and graphite felt as the cathode.”
Electrolyte selection was based on a series of compatibility tests. Gautam says, “In our studies, we found that by adjusting the counterion of specific catholyte candidates, the resulting materials can transition from being partially immiscible to completely immiscible with corresponding anolytes. This salt-out strategy proved to be very effective in developing the catholytes to work with the anolyte.”
Lithium was selected as the anode due to its high-energy density and theoretical capacity. Graphite exhibits a high specific area, porous structure, high electrical conductivity and is chemical inert.
Initial testing to evaluate the viability of this non-aqueous redox flow battery was conducted under static conditions at 27 ºC in an inert argon atmosphere. Good cycling performance with high capacity retentions above 99% were found even after cycling for 22 days. The next step was to evaluate the non-aqueous redox flow battery under flowing conditions. Figure 2 shows the battery set-up with the anolyte placed above the catholyte which is flowing into and out of a reservoir. Electrons flow from the anode on top of the battery to the cathode on the bottom.
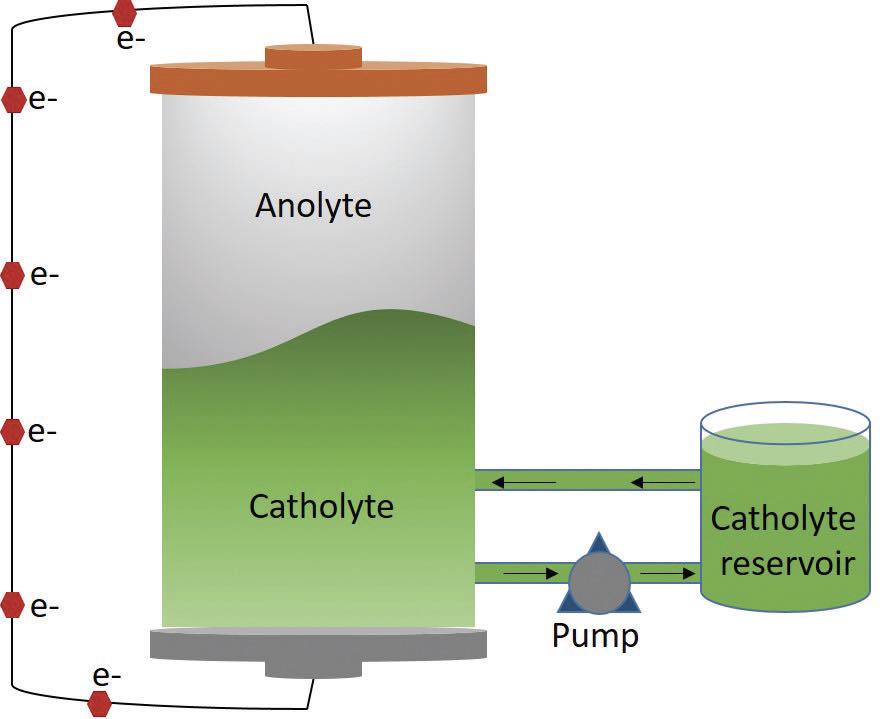 Figure 2. The setup for a non-aqueous redox flow battery shows the less dense anolyte is above the catholyte, which is being pumped into and out of a reservoir. Electron flow from the anode on top of the battery to the cathode on the bottom occurs during battery operation. Figure courtesy of the University of Cincinnati.
Figure 2. The setup for a non-aqueous redox flow battery shows the less dense anolyte is above the catholyte, which is being pumped into and out of a reservoir. Electron flow from the anode on top of the battery to the cathode on the bottom occurs during battery operation. Figure courtesy of the University of Cincinnati.
Under identical conditions to the static experiment, a battery achieved a capacity retention rate of 81% over 100 cycles that took 45 days to complete. The average cell voltage was 3.45 V at a current density of 1.5 milliamps per centimeter. The average Coulombic efficiency was 96% and the energy efficiency was 82%.
Gautam says, “We found no evidence of material degradation or solvent crossover during battery operation. This system can probably be operated for at least one year without any difficulties.”
Future work will involve optimizing the cost and efficiency of the non-aqueous redox flow battery according to Gautam. He says, “We will work to optimize the flow rates to enhance mass transfer kinetics, identify better electrolytes to improve conductivity and regulate overall battery kinetics, develop an advanced cell design to reduce dead volume of the catholyte electrode and improve capacity utilization.”
Additional information can be found in a recent article
2 or by contacting Gautam at
gautamrk@ucmail.uc.edu.
REFERENCES
1.
Canter, N. (2021), “Water soluble organic catholyte for use in redox flow batteries,” TLT,
77 (12), pp. 16-17. Available at
here.
2.
Gautam, R., Wang, X., Lashgari, A., Sinha, S., McGrath, J., Siwakoti, R. and Jiang, J. (2023), “Development of high-voltage and high-energy membrane-free nonaqueous lithium-based organic redox flow batteries,”
Nature Communications, 14, Article Number 4753.