HIGHLIGHTS
•
High-density polyethylene is converted to hydrogen and graphene through a flash joule heating process.
•
Yields for the hydrogen produced by the flash joule process ranged from 52%-68% and for graphene were from 46%-63%.
•
Significantly less energy and lower greenhouse gas emission are found using this process versus other waste plastic or biomass destruction methods.
Finding pathways to produce hydrogen from sustainable raw materials is an ongoing challenge as demand for sustainable energy sources is increasing. Hydrogen is available in various forms that are characterized by a specific color based on the manufacturing process used.
Unfortunately, the main pathway for obtaining hydrogen globally involves steam methane reforming. The “grey hydrogen” produced by this method evolves 11 metric tons of carbon dioxide for every metric ton of hydrogen in a non-sustainable process.
The ideal manner for producing hydrogen is to split water. But this process is economically unfeasible, requires expensive metal catalysts such as platinum and uses nine metric tons of water for every metric ton of “green hydrogen” produced. A potential raw material option to use is waste plastic, which has a high content of hydrogen and is present in large quantities.
James Tour, T.T. and W.F. Chao Professor of Chemistry and professor of materials science and nanoengineering at Rice University in Houston, Texas, says, “Plastics, particularly those based on polyolefins such as polyethylene, are ideal sources of hydrogen because they contain two hydro– gens per carbon atom. Polyethylene and polypropylene contain 14% hydrogen atoms by weight with carbon accounting for the remaining 86%. No other hydrocarbon, with the exception of methane or ethane, can produce as much hydrogen when completely degraded.”
A recent approach for producing hydrogen from plastic utilized iron aluminum oxides in the presence of microwaves. Tour says, “While hydrogen can be produced, this method also produces low molecular weight olefins and impure carbon nanotubes due to rapid heating rates. An equal amount of catalyst to plastic by weight needs to be employed. The catalyst is expensive and can only be used for three process runs before needing to be replaced.”
The U.S. Department of Energy has the objective of producing one kilogram of hydrogen for one dollar within this decade in a 1:1:1 goal. Tour says, “In order to meet this objective, identifying a process that does not require an expensive catalyst is required.”
In a previous TLT article,
1 asphaltenes were converted to graphene using a technique known as flash joule heating that was discovered by Tour and his colleagues. Asphaltenes are combined with the conductive material, carbon black and squeezed into a quartz tube between a porous carbon electrode and a graphite electrode. Subjecting this mixture to a high voltage discharge connected to the two electrodes increased the temperature of the mixture by approximately 3,400 K in less than 50 milliseconds.
In evaluating the flash joule heating reaction, Tour noted that gas byproducts also were generated but had not been characterized. He says, “When we generated graphene, we noticed little flames similar to an acetylene torch emanating from the reactor. The assumption was made that the gases produced were hydrocarbon such as methane and ethane. But upon placement of a liquid nitrogen trap, we were only able to trap small amounts of those gases.”
Tour and his colleagues then figured the gas produced was probably hydrogen, which has a freezing point lower than liquid nitrogen. This assumption was confirmed through the use of gas chromatography-mass spectrometry analysis.
The researchers have now used flash joule heating to produce hydrogen in high yield.
Flash hydrogen
Flash joule heating was conducted using high-density polyethylene to evaluate and optimize the concept. This non-conductive plastic was placed in a quartz tube with carbon black and compressed to have a resistance between 5-10 Ohm. The two electrodes used were a pellet of copper wool and a graphite rod. The tube was then subjected to a 100-130 volt discharge for typically less than three seconds. Tour says, “Experimental results showed that conducting four consecutive flash joule procedures maximizes the yield of hydrogen up to an efficiency of 92.7%. The purity of hydrogen gas isolated was found to be 87% when the initial resistance is 6 Ohm.”
The researchers designated the hydrogen produced in this manner as “flash hydrogen.”
Initial experiments found that methane and low molecular weight alkenes were isolated in the gas stream with hydrogen. The residue contained graphene and higher molecular weight hydrocarbons. Tour says, “As we raised the peak temperature, increased the heating rate and generated shorter discharge durations, hydrogen yield increased, and the residue was almost entirely graphene.”
The graphene obtained was characterized through a number of techniques including Raman spectroscopy and bulk powder X-ray diffraction. A scanning electron micrograph of crystalline graphene produced from flash joule heating is shown in Figure 2. Graphene has been found to be useful in a number of applications including lubricants.
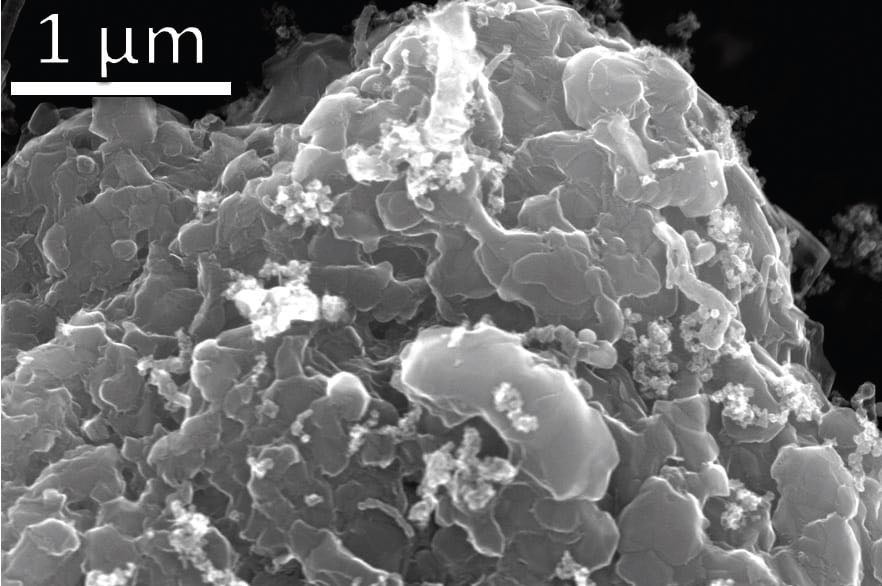 Figure 2. Layered stacks of nano-scale flash graphene sheets are formed during the flash joule heating of waste plastic. Figure courtesy of Rice University.
Figure 2. Layered stacks of nano-scale flash graphene sheets are formed during the flash joule heating of waste plastic. Figure courtesy of Rice University.
In evaluating waste plastic streams, the researchers were able to directly treat streams with flash joule heating without doing any separation or washing. Yields for flash hydrogen ranged from 52%-68% and for graphene were from 46%-63%.
Tour says, “The polymers probably undergo a homolytic decomposition procedure that literally breaks carbon-hydrogen bonds. Under the reaction conditions, the most thermodynamically stable products, hydrogen and graphene, are produced.”
The sustainability of producing flash hydrogen was evaluated using a life-cycle assessment that compared to manufacture of hydrogen by other techniques. Approximately 33%-95% less energy and 65%-89% lower greenhouse gas emission are found with the manufacture of flash hydrogen versus other waste plastic or biomass destruction methods. A determination was made that flash hydrogen produced 84% less greenhouse gas emission than steam methane reforming and required 4% less energy than what is used in generating green hydrogen from water splitting.
Tour says, “The next step is for developing methods for scaling-up flash joule heating of waste plastic so that it becomes commercially viable.”
A life-cycle assessment of the rapid flash joule heating of waste plastic demonstrates that hydrogen produced by this process has a price of $-4.30 per kilogram for each kilogram of hydrogen produced even if the graphene produced is sold at 5% of its current price. This surpasses the U.S. Department of Energy goal of $1 per kilogram.
Additional information can be found in a recent article
2 or by contacting Tour at
tour@rice.edu.
REFERENCES
1.
Canter, N. (2023), “Sustainable conversion of asphaltenes to graphene,” TLT,
79 (4), pp. 14-15. Available
here.
2.
Wyss, K., Silva, K. Bets, K., Algozeeb, W., Kittrell, C., Teng, C., Choi, C., Jacob, W., Beckham, J., Yakobson, B. and Tour, J. (2023), “Synthesis of clean hydrogen gas from waste plastic at zero net cost,”
Advanced Materials, available
here.