HIGHLIGHTS
•
Zinc-air batteries operate through two redox reactions at the cathode and potentially exhibit three times the energy density of a lithium-ion battery.
•
Catalysts based on a sub-class of metal organic frameworks and a layered double hydroxide derivative work synergistically to facilitate oxygen-evolution and oxygen-reduction reactions.
•
The zinc-air battery exhibits a high power density of 22 milliwatts per cubic centimeter, and stability for over 950 hours.
The move to electrification has accelerated development of different battery technologies for applications such as electric vehicles. Currently, the most widely used type in electric vehicles is lithium-ion batteries, but there are concerns about safety when they are used.
In a previous TLT article,
1 researchers developed a lithium-air battery that has the potential to exhibit an energy density well beyond what is possible for lithium-ion batteries. The lithium-air battery contains lithium at the anode, which reacts with oxygen from the air at the cathode to produce energy. This process generates lithium oxide through a four-electron pathway that may boost energy density by as much as four times above lithium-ion batteries at room temperature. Experimental results confirm that generation of lithium oxide during battery discharge produced an average electron-to-oxygen ratio of 3.96, close to the theoretical ratio of 4.
Dr. Muhammad Rizwan Azhar, lecturer, founding member of the Mineral Resource Recovery Center in the School of Engineering at Edith Cowan University in Joondalup, Australia, says, “Another battery technology, containing zinc as the anode and oxygen as the cathode, is under evaluation, because potentially it can exhibit three times the energy density of a lithium-ion battery. Rechargeable zinc-air batteries operate through two oxygen redox reactions at the cathode. During charging, the oxygen-evolution reaction takes place while, during discharging, the oxygen-reduction reaction occurs. Other attractive features include low cost, environmentally friendly and inherent safety in comparison to a lithium-ion battery, because the zinc-air battery operates in an aqueous environment.”
Azhar points out that zinc is prevalent in Australia with the country having the largest reserves in the world that account for 20% of the total globally. Unfortunately, rechargeable zinc-air batteries have not become a viable technology due to subpar performance caused by sluggish oxygen-evolution and oxygen-generation reaction kinetics at the cathode. This causes a large voltage gap during the charging/discharging cycle producing low efficiency.
Azhar says, “The challenge for us was to find catalysts specific for oxygen-evolution and oxygen-reduction that are highly active and durable. Since these two reactions take place at different active sites and proceed through different reaction pathways, it is difficult to find one catalyst that will be able to effectively facilitate both oxygen-evolution and oxygen-reduction.”
Azhar and his colleagues have now developed a nanocomposite catalyst that has separate active sites for facilitating the oxygen-evolution and oxygen-reduction reactions.
LDH – Co-N-C catalyst
The nanocomposite catalyst is based on a cobalt-coordinated, nitrogen-rich and porous carbonaceous frame (Co-N-C) that is produced by pyrolysis of ZIF-67, a sub-class of metal organic frameworks (MOFs), and a layered double hydroxide (LDH) derivative.
Azhar says, “We have been studying MOFs for several years to better understand their catalytic nanostructure. One MOF derivative, ZIF-67, can be used to produce CO-N-C, which exhibits a large surface area, excellent electrical conductivity and, most importantly, can effectively catalyze the oxygen-reduction reaction.”
LDHs are based on two-dimensional lamellar crystals that contain bivalent and trivalent cationic host layers with hydroxide anions placed between the layers to provide hydrogen bonding with water molecules and interact with intercalated anions (such as carbonate). Azhar says, “This class of compounds has been found to effectively catalyze the oxygen-evolution reaction. In particular, catalyzing the conversion of hydroxyl ions to oxygen and water works very well if nickel and iron are used as the cations. But this LDH catalyst still displays low electronic conductivity. To improve this characteristic, we introduced cobalt as a third cation and found, as a consequence, that the LDH catalyst displayed significantly better electronic conductivity.”
A hydrothermal process was used to produce the nanocomposite catalyst by treatment of Co-N-C with iron and nickel cations at 120 C for 14 hours. During the reaction, cobalt (+2) cations were etched from the surface of the Co-N-C and further oxidized to cobalt (+3) cations, which formed a thin layer of a cobalt, nickel and iron LDH on the surface of the Co-N-C.
A schematic showing how the zinc-air battery operates with the nanocomposite catalyst is in Figure 1. The electrolyte used by the researchers is 6 molar potassium hydroxide. Included in the schematic are the motions of the electrons and the hydroxyl ions in the electrolyte.
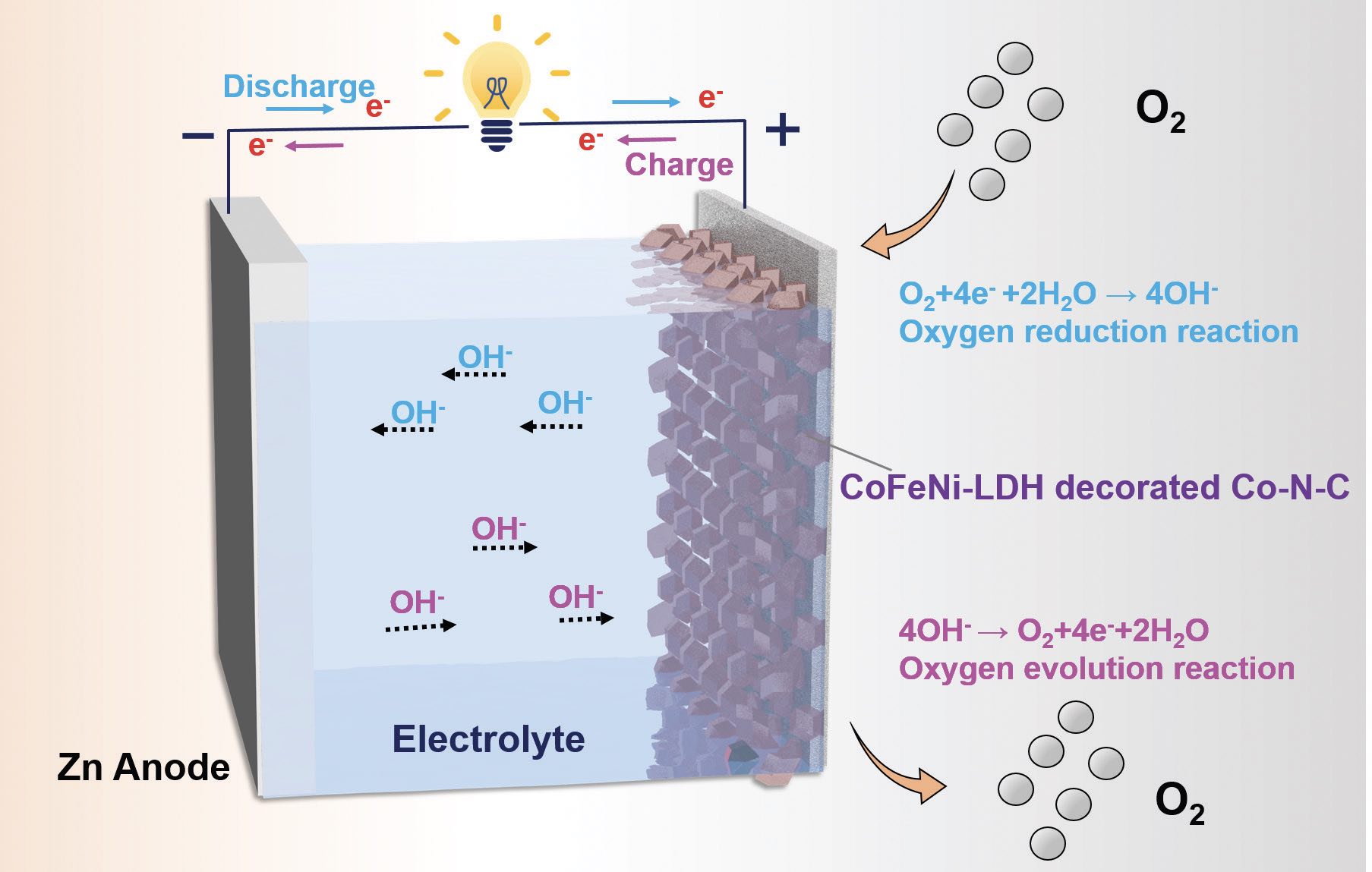 Figure 1. The operating scheme of a zinc-air battery is shown, which includes the flow of electrons, flow of hydroxyl ions and the two reactions (oxygen-reduction and oxygen-evolution) that occur at the cathode. Figure courtesy of Edith Cowan University.
Figure 1. The operating scheme of a zinc-air battery is shown, which includes the flow of electrons, flow of hydroxyl ions and the two reactions (oxygen-reduction and oxygen-evolution) that occur at the cathode. Figure courtesy of Edith Cowan University.
Use of the nanocomposite catalyst in the zinc-air battery leads to a small voltage gap of 0.77 V at 5 milliamperes per square centimeter, a high peak power density of 22 milliwatts per cubic centimeter and stability for over 950 hours. Azhar says, “The results from our work show that the Co-N-C and LDH catalysts work synergistically in catalyzing the oxygen-reduction and oxygen-evolution reactions, respectively.”
The authors, having now conducted a proof of concept for using the nanocomposite catalyst in the zinc-air battery, are looking to evaluate this approach in real-world applications. Azhar says, “We believe this type of battery will be very useful in storing energy. This is important to supplement solar and wind energy. In our region in Australia, there are communities that are in rural locations which could use this energy storage technology to ensure a constant supply of electricity.”
The zinc-air battery also may be potentially used in powering electric vehicles. Additional information can be found in a recent article
2 or by contacting Azhar at
m.azhar@ecu.edu.au.
REFERENCES
1.
Canter, N. (2023), “Lithium-air battery,” TLT,
79 (6), pp. 32-33. Available
here.
2.
Arafat, Y., Zhong, Y., Azhar, M., Asif, M., Tadé, M. and Shao, Z. (2023). “CoNiFe-layered double hydroxide decorated Co-N-C network as a robust bi-functional oxygen electrocatalyst for zinc-air batteries,”
EcoMat; e12394.