TLT: You have worked in the field of tribology for more than 40 years. Can you reflect on your journey in this field over the years and your involvement with STLE?
Hsu: The 1970s was a transition period in our understanding of tribology, prompted by space exploration, high compression engine development and an influx of new materials into tribology for higher performance and space exploration. The fundamental relationship between material property and the ability to successfully lubricate the new material systems emerged. Some materials work fine under conventional lubrication, and some do not. This presents a seemingly unsolvable puzzle. Why does a lubricant work on steel but does not work on ceramics? The space equipment also encountered extreme temperatures in space. The U.S. Army is responsible for issuing lubricant specifications for vehicles used by the Army. Facing the lack of fundamental understanding of lubrication, these groups gather together to define the gaps of knowledge in lubrication. The results were published in a book,
1 which highlighted the gaps in our understanding of lubrication mechanisms and the interactions between lubricants and various materials. This publication lays out a roadmap for future lubrication research.
Among the gaps identified in this book, two items attracted my attention: 1.) What is the real temperature at the boundary lubricating interface? 2.) What is the nature of lubricating film? How are they formed and how do they work? No. 1 became my thesis. Keying on the discovery of organo-iron in lubricant oxidation results, I developed a ppm-sensitive trace analysis technique to track organometallic compound formation on sliding surfaces, and coupled with molecular weight measurements, using an kinetic model, I was able to measure the actual reaction temperatures to generate the amount of organometallic compounds. The interfacial reaction temperature was estimated at 351 C for 40 Kg load, 600 rpm and 75 C bulk oil temperature.
The work
2 earned the STLE Captain Alfred E. Hunt Memorial Award in 1980 for the best paper in lubrication of the year. This was one of my first experiences with STLE, the home of the lubrication community.
I joined STLE in 1978 and have participated in paper solicitation, initiated new technical groups (tribochemistry, nanotribology, analytic methods and others), was chair of the 1987 STLE Annual Meeting Planning Committee and served on the STLE Board of Directors from 1987 to 1994. Over the years, I have received most of the STLE awards while pursuing the lubricating film formation and their strength.
3,4
Lubrication involves materials, base oils, additives, tribochemistry and fluid dynamics. STLE, as a single organization, has all these experts working on various aspects of lubrication. This is rare, and as my career grows, I move to other areas such as ceramics, surface engineering, nanotechnology and fuel economy. But my primary focus is still on lubrication mechanism. A key question I often ask myself is: Is the current lubrication level of understanding close to the final level of lubrication technology? The basic science of lubrication appears to be stagnant for a relatively long time. Most of the additives were invented 40 or 50 years ago. Could self-repairing lubricants or permanent lubrication for life be possible?
TLT: Can you please discuss a specific challenge/research question that you found most satisfactory to tackle?
Hsu: Lubrication science basically stems from fluid dynamics and chemicals interacting with sliding surfaces under load. Tribochemistry, triboelectricity and surface topography all play a role in the outcome, resulting in friction control and robust durability. Lubrication starts out as a complex amalgamation of a single purpose, yet how many avenues have we not touched or do not understand their potential? Can materials achieve self-lubrication by themselves? Can lubricant additives be able to be stored within the surface to resupply the surface to maintain lubrication? Can we develop perpetual self-healing lubrication chemistry? Would we really need liquid petroleum base oils to achieve lubrication?
Technology often rises out of the basic knowledge of science. To project future technological development, we need to understand the basic science of lubrication. I started looking into some fundamental issues: What is the colloidal dispersion of molecules in oil? How does a lubricant molecule change under shear pressure? What is the near surface molecular arrangements of additives and lubricant components? These fundamental questions prompted me to look for answers using advanced instruments such as synchrotron quantum beams, a Raman laser, Fourier transform infrared (FTIR) spectroscopy and some homemade instruments to examine simple molecules, such as stearic acids and oleic acids.
The use of new instruments and methods was able to show that stearic acid adsorbed on the copper surface shifted to more robust two prone bonding by adding bonds to the oxygen under shear. So, both shear and higher temperatures produced more robust bonding with the surface. For reverse micelle in solution in the presence of a sliding surface, we found the molecules (additives) tend to align close to the sliding surface with polar heads toward the surface
(see Figure 1a and b). The most polar species tend to be closest to the surface. The packing order tends to be influenced by the tribochemical reaction products produced at the surface. Besides our results, cryogenic scanning electron microscopy (SEM) results tend to confirm this observation. The results from these studies heavily influence my thinking and lead to the development of measuring lubricating film shear strength studies to produce more robust lubricating films.
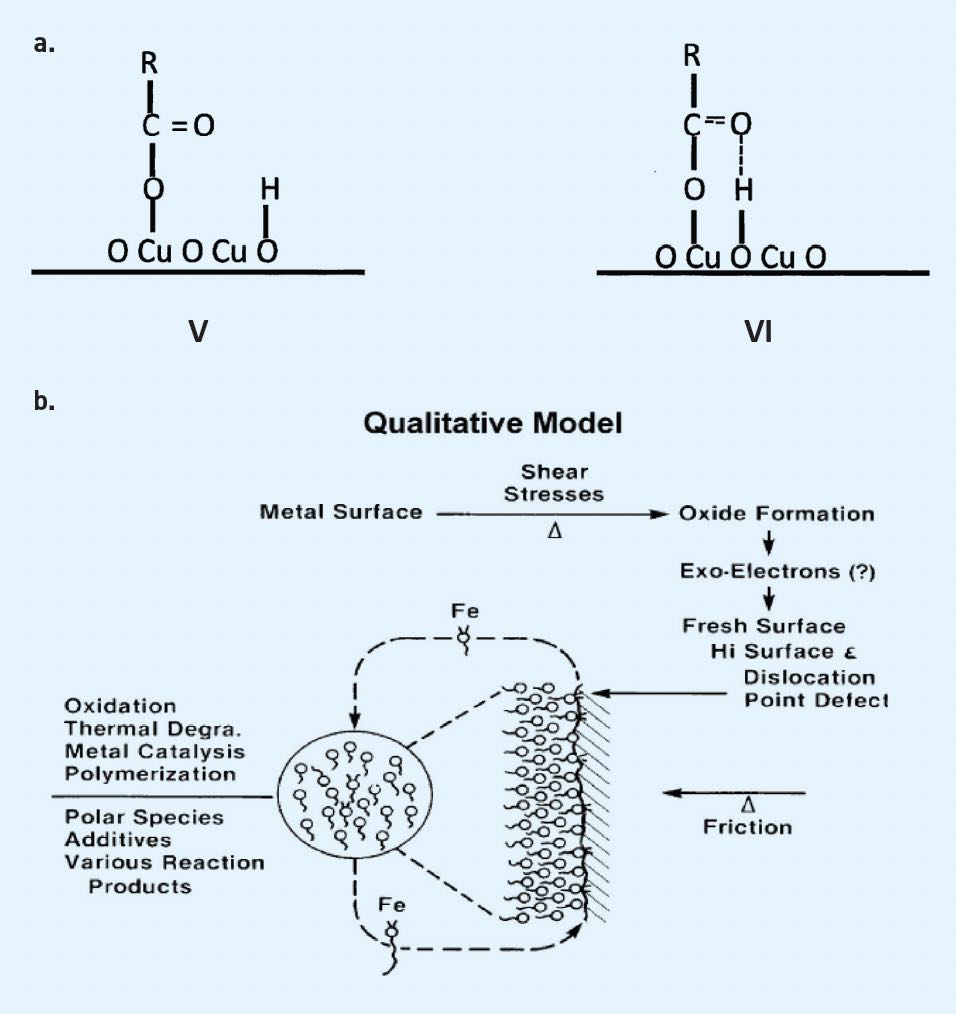 Figure 1. a.) Molecular rearrangement due to shear action, from single point attachment (monodente) to two attachment points (bidente) strengthening the adsorption bonding. Figure courtesy of Ref. 4. b.) Polar molecules under boundary lubrication conditions tend to adsorb onto the rubbing surface according to polarity and reactivity at steady state. Figure courtesy of Ref. 5.
Figure 1. a.) Molecular rearrangement due to shear action, from single point attachment (monodente) to two attachment points (bidente) strengthening the adsorption bonding. Figure courtesy of Ref. 4. b.) Polar molecules under boundary lubrication conditions tend to adsorb onto the rubbing surface according to polarity and reactivity at steady state. Figure courtesy of Ref. 5.
TLT: Can you elaborate on your research in friction polymers and the role of lubricant chemistries in delivering effective lubrication?
Hsu: The nature and origin of the brown film left on the wear scar has been my unyielding pursuit from the very beginning. Based on the ultra-sensitive atomic absorption analysis, there are organo-metal species in the surface film. But we have no idea about the composition and molecular weight. There was an analytical technique using gel permeation chromatography (GPC) to identify polymer molecular weight. We have been using an ultrasensitive graphite furnace atomic absorption (GFAA) to measure the organic metals in the film. If we can couple these two instruments together, as the GPC separates various molecular weight fractions, the sampling stream is immediately passed through the GFAA, then we can measure the metal species in the molecular weight fraction. After many trials, we successfully coupled the two instruments to measure the molecular weight and metal concentration of the surface film extract. These are minute quantities, so the measurements have to be done under a quiet, clean and stable environment with constant voltage regulation.
Figure 2a is oxidation of lubricant on iron/cu disks. The topline is the molecular weight (MW) as a function of time. Underneath is the metallic signal. The top graph in Figure 2b is from an actual wear test of the lubricant showing friction polymer formation until the molecular weight reaches 1,000,000 MW, the limit of solubility. The midline in Figure 2b is from the used oil from ASTM III reference engine oils, showing very high friction polymer concentration.
6 Subsequent studies reveal that these reactions are predominantly from transition metals series where coordination chemistry has lower activation energies. Base oils form the majority of soft polymeric products and the antiwear additives form tenacious surface films to resist wear. Successful lubrication requires both materials to be present.
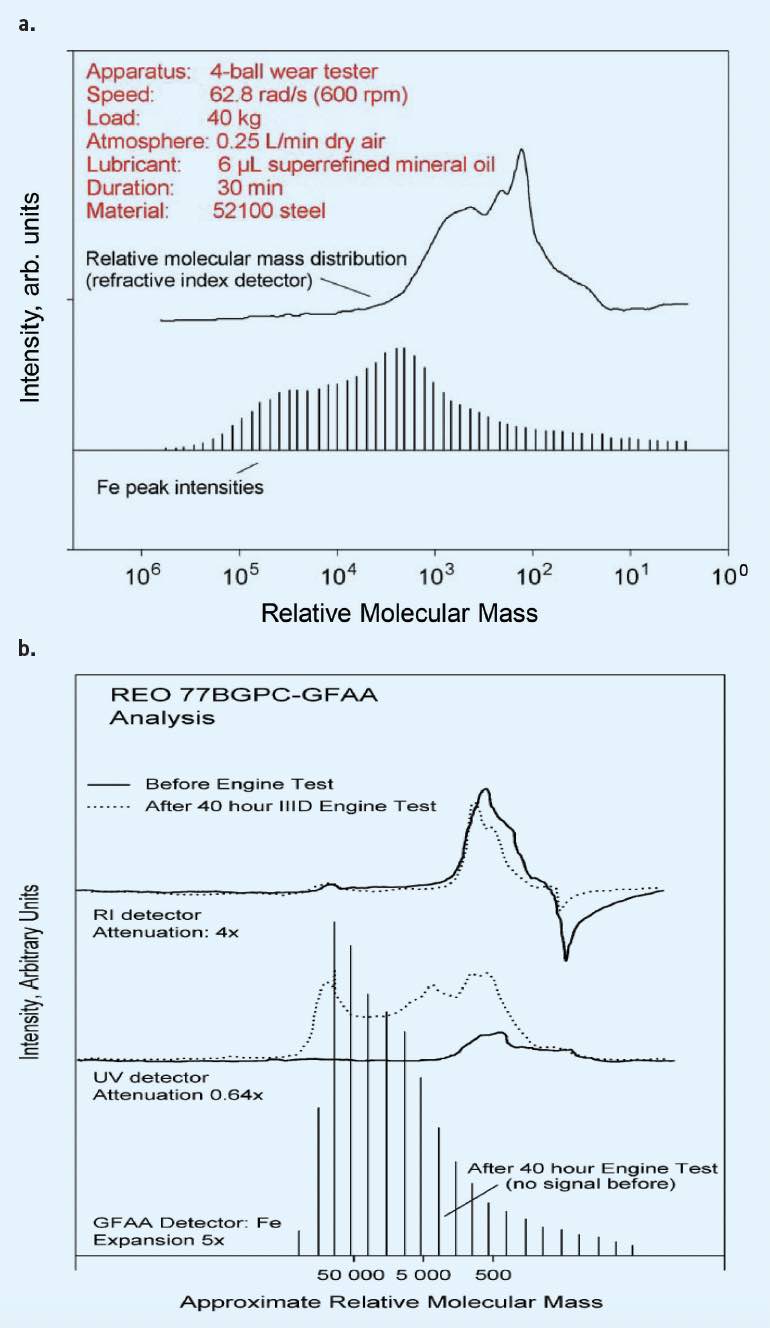 Figure 2. a.) Organometallic compounds measurement as a function of time and molecular weight in a four-ball wear test. The surface reaction products are extracted using solvent and separated by gel permeation chromatography and metal analysis by atomic absorption. b.) Similar organometallic compounds were found when extracted from on engine parts conducting the ASTM III sequence tests using Reference oil REO 77B. Figure courtesy of Ref. 6.
Figure 2. a.) Organometallic compounds measurement as a function of time and molecular weight in a four-ball wear test. The surface reaction products are extracted using solvent and separated by gel permeation chromatography and metal analysis by atomic absorption. b.) Similar organometallic compounds were found when extracted from on engine parts conducting the ASTM III sequence tests using Reference oil REO 77B. Figure courtesy of Ref. 6.
When we move into other classes of materials, to our surprise, even insulators and semiconductor materials form similar organometallic reactions under heat or sliding,
7 but the structures are somewhat different with distinct bonding structures and need higher temperatures and speed/ load to form, but the end point is organometallic formation with increasing molecular weights.
This discovery has large implications in terms tribology, corrosion, rusting and other forms of material degradations.
TLT: As we progress to advanced materials and manufacturing, characterization techniques and computing capabilities, where do you see the field going within the next five to 10 years?
Hsu: The world is changing rapidly. Global warming effects (severe weather storm, fire, etc.) will increase in intensity; the U.S. suffered $80 billion in damages due to storms and fires in 2020. Electrification of the transportation sector has been agreed to by world leaders, and electric vehicles will start to replace internal combustion engines in the next 10 years. Autonomous systems aided by artificial intelligence (AI) and machine learning (ML) are evolving rapidly. Depleting natural resources are pushing the development of sustainable mechanical systems. So, the future will demand robust, durable systems and, if possible, self-sustaining and environmentally friendly systems.
From a tribology and lubrication perspective, some new developments are helping the area to move toward these goals: 1.) The use of surface texturing8 can alter the basic lubrication mechanism, providing reserve of lubricating species inside the textural features at the interface, helping to dissipate thermal stresses and moderate fatigue processes. 2.) Recent development of microencapsulated lubricant and additives will significantly prolong the lubricant life and use much less lubricant in semi-dried systems.
Some have said the 21st century will be the century of thermal science development in relatively dry and hot electrical systems. 3.) Increasingly robust wear resistant materials are being developed, such as thermal spray hard coatings on engine ring surfaces. Even though the cermet material is non-reactive to lubricant or additives, the hard composite with in situ discrete texture formation during sliding really does not need conventional lubricants. 4.) The development of microcapsules containing mixtures of additives and nanoparticles has the potential to achieve limited self-healing capability. The microcapsules can hide in the surface textured surface layer and release needed lubricating species buried at various depths near the surface and can potentially extend the lubrication effective range tremendously
(see Figure 3a and b).
 Figure 3. a.) A microcapsule can contain multiple smaller separate capsules containing either the same chemicals or one other different chemical. b.) To facilitate graduate additive release, through-holes can be fabricated into the microcapsule wall. Figure courtesy of Ref. 9.
Figure 3. a.) A microcapsule can contain multiple smaller separate capsules containing either the same chemicals or one other different chemical. b.) To facilitate graduate additive release, through-holes can be fabricated into the microcapsule wall. Figure courtesy of Ref. 9.
One question that remains in moving forward toward 100% dry lubrication for life is the ability to perform equally the cleaning power of conventional lubricants, which carry the sludge, varnish and wear debris effectively away from the contact. But if in a dry lubricated system, there may not be that much sticky waste generated. Air jet cleaning may work in most cases. Not using oil/lubricants would indeed be environmentally friendly, and we really do not need frequent oil change intervals. So, there could be revolutionary changes awaiting us in the future.
TLT: What advice would you give to young researchers and students working in this area of research?
Hsu: Technology today moves fast with less than three years of a technology cycle. Successful students and researchers should have an open mind and be ready and prepared to learn new things. The idea of belonging to a specific discipline based on outdated departmental boundaries is gone. In tribology, for example, one has to have basic knowledge of surfaces/materials, lubricant viscometry, contact mechanics, fluid dynamics and additive chemistry used to prevent rust, corrosion and fatigue. If one only focuses on one subject, it will soon become self-limiting to solve complex multidisciplinary challenges.
REFERENCES
1.
Ling, F., Klaus, E. and Fein, R., eds. (1969),
Boundary lubrication: An appraisal of world literature, ASME Research Committee on Lubrication.
2.
Hsu, S. and Klaus, E. (1978), “Estimation of the molecular junction temperatures in four ball contacts by chemical reaction rate studies,”
ASLE Transactions, 21 (3), pp. 201-210.
3.
Fischer, D., Hu, Z. and Hsu, S. (1997), “Molecular orientation and bonding of mono- layer stearic acid on a copper surface prepared in air,”
Tribology Letters, 3, pp. 41-45.
4.
Hu, Z., Hsu, S. and Wang, P. (1992), “Tribochemical and thermochemical reaction of stearic acid on copper surfaces studied by infrared microspectroscopy,”
Tribology Transactions, 35 (1), pp. 189-193.
5.
Hsu, S. (2004), “Molecular basis of lubrication,”
Tribology International, 37 (7) pp. 553-559.
6.
Hsu, S. and Gates, R. (2005), “Boundary lubricating films: Formation and lubrication mechanism,”
Tribology International, 38 (3) pp. 305-312.
7.
Hsu, S. and Gates, R. (2006), “Effect of materials on tribochemical reactions between hydrocarbons and surfaces,”
J. of Phys. D; Applied Physics, 39, 3128-3137.
8.
Hsu, H., Jing Y., Hua, D. and Zhang, H. (2014), “Friction reduction using discrete surface textures: Principle and design,”
J. of Phys. D: Applied Physics, 47, 335307.
9.
Hsu, S. and Zhao, F. (March 16, 2017), “Microencapsulation of chemical additives,” U.S. patent 2017/0073610 A1.