HIGHLIGHTS
•
A new lithium-air battery has been developed that uses a four-electron reaction involving the formation of lithium oxide to generate an energy density that exceeds the current capability of a lithium-ion battery.
•
formation of lithium oxide at the cathode is an important reason for why the lithium-air battery performs so well.
•
The presence of a solid electrolyte is important to ensure that a four-electron reaction needed to produce lithium oxide occurs during operation of the battery.
Battery development is continuing, and significant progress has been made in increasing energy density while reducing cost. But challenges remain with the widely used lithium-ion battery particularly due to the formation of needle-like structures known as dendrites during operation that, if not inhibited, can lead to a short circuit and potentially a fire.
In a previous TLT article,
1 researchers used a microfluidic-based electrochemical system to measure the growth of dendrites. By evaluating the rate of cross-flow of ions during deposition on a battery cathode, a determination was made that dendrites can be formed from electroconvective vortexes. As ion flow rate increases, the researchers found that vortex formation declined minimizing the potential for dendrites to grow. Instead, small, uniform crystal bumps were detected on electrodes with diameters much smaller than vortex particles.
As discussed in this article, other battery types besides lithium-ion also are under development with researchers working to identify other technologies that produce higher energy densities but are safer to use. Dr. Larry Curtiss, distinguished Fellow at Argonne National Laboratory in Lemont, Ill., says, “A technology that is showing potential for commercialization is a lithium-air battery. In contrast to other battery types that rely on the rapid movement of ions in an intercalation process, a lithium-air battery generates energy through chemical transformation that involves the forming and breaking of chemical bonds.”
In a lithium-air battery, lithium ions originating at the anode react with oxygen from the air at the cathode to produce energy. Past work has involved the reaction of lithium cations with oxygen to form either lithium peroxide in a two-electron reaction or disproportionation of the intermediate, lithium superoxide, which is formed as part of a one-electron reaction. The reverse process during charging results in the conversion of either lithium peroxide or lithium superoxide back to lithium and oxygen.
A better approach to produce a lithium-air battery is to figure out a pathway that can start with lithium ions and oxygen and produce lithium oxide. Curtiss says, “Formation of lithium oxide is a four-electron reaction that has the potential to boost the energy density by as much as four times above lithium-ion batteries at room temperature. The challenge to achieve this result is that an oxygen–oxygen bond must be broken during discharge and the bond reformed during the charging process.”
Due to the difficulties in breaking and reforming oxygen bonds as the battery cycles, there has not been much success in the lithium oxide pathway. A new approach has now been reported that leads to the preparation of a lithium-air battery using a lithium oxide pathway during discharge.
In situ cathode formation
Curtiss and his colleagues have reported a new lithium-air battery that utilizes lithium oxide formation in a four-electron reaction that can now work up to a capacity of approximately 10.4 milliamps per cubic centimeter. This results in a specific energy density of approximately 685 watt-hours per kilogram of the cell.
A unique aspect of the battery is the use of a solid ceramic polymer electrolyte (CPE) based on nanoparticles of a lithium germanium phosphorus sulfur compound, known as LGPS, that are placed in a matrix containing {3-[methoxy(polyethyleneoxy)6-9 propyl] trimethoxysilane} (mPEO-TMS). Curtiss says, “Our colleague, Mohammad Asadi, professor in the department of chemical and biological engineering at the Illinois Institute of Technology in Chicago, Ill., recognized that LGPS has a very high ionic conductivity, but this can lead to instability due to the reactive sulfur. Placement of the TMS functionality in the composite solid electrolyte enables the silicon atoms to coordinate with the sulfur atoms stabilizing LGPS.”
The discharge/charge process for the lithium-air battery is shown in Figure 2. During the discharge phase, lithium ions flow from the anode through the CPE to the cathode where a reaction takes place with oxygen from the air and electrons from the cathode to produce lithium oxide. To complete the cycle, during charging, an oxygen molecule is reformed from lithium oxide, and the resulting lithium ions move back to the anode.
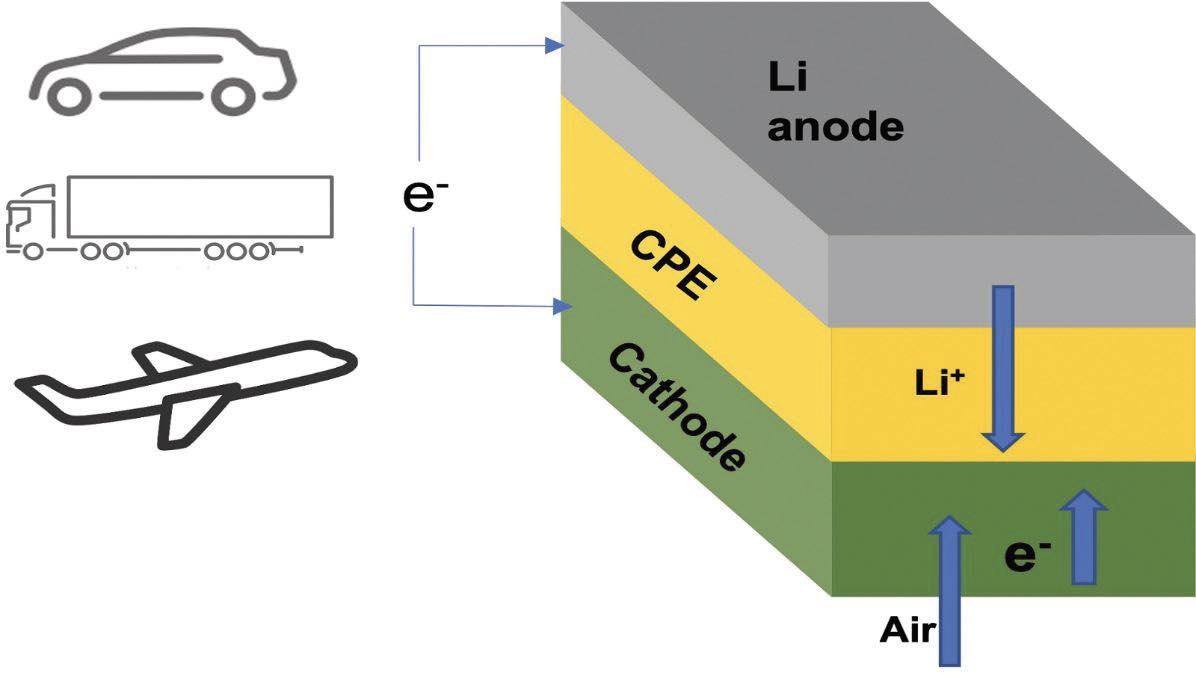 Figure 2. The flow of lithium ions (Li+), electrons (e–) and air (oxygen) in a lithium-air battery is shown. CPE is a solid ceramic polymer electrolyte that is an important component in the battery. Potential applications include electric vehicles. Figure courtesy of Argonne National Laboratory.
Figure 2. The flow of lithium ions (Li+), electrons (e–) and air (oxygen) in a lithium-air battery is shown. CPE is a solid ceramic polymer electrolyte that is an important component in the battery. Potential applications include electric vehicles. Figure courtesy of Argonne National Laboratory.
The cathode used at this stage of the project is trimolybdenum phosphide nanoparticles on a hydrophobic gas diffusion layer in an environment containing nitrogen (78%) and oxygen (21%) that simulates the Earth’s atmosphere. Curtiss says, “The cathode is not special to the lithium-air battery. The significant aspect is that
in situ formation of lithium oxide produces cathode material on top of the cathode. For the lithium-air battery, the cathode must exhibit good electrical conductivity and have the right morphology (roughness) to make room for the discharge product.”
Titration and ultraviolet-visible spectroscopy experiments were conducted to determine if lithium oxide was produced during battery discharge. Titration data found that the average electron to oxygen ratio is 3.96, which is quite close to the theoretical ratio of 4. This result demonstrates that lithium oxide is the predominant substance formed. Spectral analysis also indicates that lithium oxide is consumed during the reversibility of the lithium-air battery during its operation.
An experiment comparing the performance of the lithium-air battery with a similar battery having a liquid electrolyte shows the unique aspect of using the solid electrolyte. Curtiss says, “We found that the liquid electrolyte battery system cycles using a two-electron process that favors the formation and decomposition of lithium peroxide. This is due to the need for lithium ions to be desolvated and for the oxygen to be displaced in the liquid electrolyte.”
The researchers demonstrated that the lithium-air battery with the CPE can run at high performance for 1,000 cycles showing good durability. Curtiss says, “More work needs to be done to increase the capacity of the lithium-air battery and scale it up for commercialization.” The researchers believe a lithium-air battery can be produced that has a specific energy of greater than 1 kilowatt hour per kilogram, which represents a volumetric energy density of approximately 1,000 watt-hours per liter. This energy density is much beyond what is possible for lithium-ion battery technology.
Additional information can be found in a recent article
2 or by contacting Argonne National Laboratory at
media@anl.gov.
REFERENCES
1.
Canter, N. (2021), “Inhibition of dendrite formation: Ion flow,” TLT,
77 (6), pp. 32-33. Available
here.
2.
Kondori, A., Esmaeilirad, M., Harzandi, A., Amine, R., Saray, M., Yu, L., Wen, J., Shan, N., Wang, H., Ngo, A., Redfern, P., Johnson, C., Amine, K., Yassar, R., Curtiss, L. and Asadi, M. (2023), “A room temperature rechargeable Li
2O-based lithium-air battery enabled by a solid electrolyte,”
Science, 379 (6631), pp. 499-505.