HIGHLIGHTS
•
Splitting water into hydrogen and oxygen is quite challenging, particularly if sea water is used because the presence of chloride anions may corrode metallic electrodes and interfere with the oxygen evolution reaction.
•
A nitrogen-doped nickel molybdenum phosphide catalyst has been found to produce hydrogen at low overpotentials with a current density better than commercial catalysts.
•
When used as both the cathode and the anode, the nitrogen-doped nickel molybdenum phosphide catalyst displayed a maximum efficiency of 95%.
Decarbonization has become an ongoing process in the effort to move away from processes that rely on hydrocarbons. Utilization of hydrogen is emerging as a viable option due to the potential for this element to be used in power generation, fuel cells and industrial applications. The ultimate objective is to develop a process to cost efficiently manufacture green hydrogen by splitting water in an electrolysis process.
A past TLT article
1 discusses a process for producing hydrogen at ambient temperature through the interaction of gallium with aluminum in the presence of water. The researchers pressed gallium into an aluminum foil resulting in the formation of a composite. After introduction of deionized water, a reaction instantaneously occurred leading to the formation of hydrogen gas. A high yield of 90% hydrogen can be achieved. Different sources of water including tap water, rainwater, recycled water and sea water were used. In all cases except for sea water, hydrogen gas was produced.
This example is representative of the work done to find a cost effective process for producing hydrogen. The challenge is how to develop a catalyst that will split sea water. With the limited global reserves for fresh water, finding an approach to use sea water, which represents 96.5% of the world’s total, is paramount. Desalination is an option, but this process is very energy intensive and also may end up with a positive carbon footprint, which is the opposite of what is needed.
Dr. Nasir Mahmood, a vice-chancellor’s senior research Fellow in the School of Science at RMIT University in Melbourne, Australia, says, “The complex nature of sea water makes it very difficult to find a suitable catalyst that will efficiently produce hydrogen and oxygen and be durable. Sea water contains many components, including biological life, solid waste and many different types of minerals.”
The single biggest concern for Mahmood is the presence of chloride anions in sea water. He says, “Besides corroding metallic electrodes, chloride can produce elemental chlorine in the chlorine evolution reaction, which will interfere with the oxygen evolution reaction at the anode. Moving to alkaline pH minimizes the chlorine evolution reaction, but at high overpotentials, there is concern about producing hypochlorite.”
Mahmood also points out that kinetics of the hydrogen evolution reaction at the cathode also can interfere with splitting water. The objective in working with sea water is to find a catalyst that will be effective for both the oxygen evolution and hydrogen evolution reactions at low chlorine evolution reaction.
Researchers have now identified and evaluated a potential new catalyst that meets these requirements.
Nitrogen-doped nickel molybdenum phosphide
Mahmood and his colleagues produced a nitrogen-doped nickel molybdenum phosphide catalyst that efficiently splits sea water into hydrogen and oxygen without any negative side reactions and without loss of activity over time. He says, “Transition metal phosphides are one of the best catalyst classes for splitting water. The problem in working with them is their stability. Initially, they exhibit excellent properties similar to noble metals, but over time the surface of the catalyst will change due to side reactions such as oxidation and specific components derived from the catalyst may end up dissolving in water.”
The most stable of the phosphides is the one where the transition metal is molybdenum. Mahmood says, “We found that by modifying the molybdenum phosphide through the introduction of nickel and nitrogen, the surface properties of the catalyst change leading to an improvement in electrical conductivity. This is achieved through tuning the metal–non-metal bond lengths.”
The researchers synthesized the catalyst in a two-step process starting with a hydrothermal process conducted at 200 C for 24 hours that produces two-dimensional porous sheets, which followed by phosphorization at 400 C for three hours under a nitrogen atmosphere, yielded the nitrogen-doped nickel molybdenum phosphide catalyst.
Initial testing was conducted by evaluating a sample of sea water taken from a beach near RMIT University in Melbourne. Mahmood says, “We took the sample, filtered out large pieces of waste, adjusted the pH into the alkaline range (using one molar potassium hydroxide) and conducted linear sweep voltammetry. The results show that the catalyst exhibits excellent activity in converting sea water to hydrogen at low overpotentials. A current density of 10 milliamps per centimeter was found, which is better than a commercial platinum/carbon catalyst.”
Full water splitting was conducted with the nitrogen-doped molybdenum phosphide used as both the cathode and anode
(see Figure 1). Excellent results were achieved at ambient temperature under alkaline pH conditions for both the hydrogen evolution and oxygen evolution reactions. Mahmood says, “We determined that the catalyst displayed a maximum efficiency of 95% and showed excellent durability when evaluated for 24 hours.”
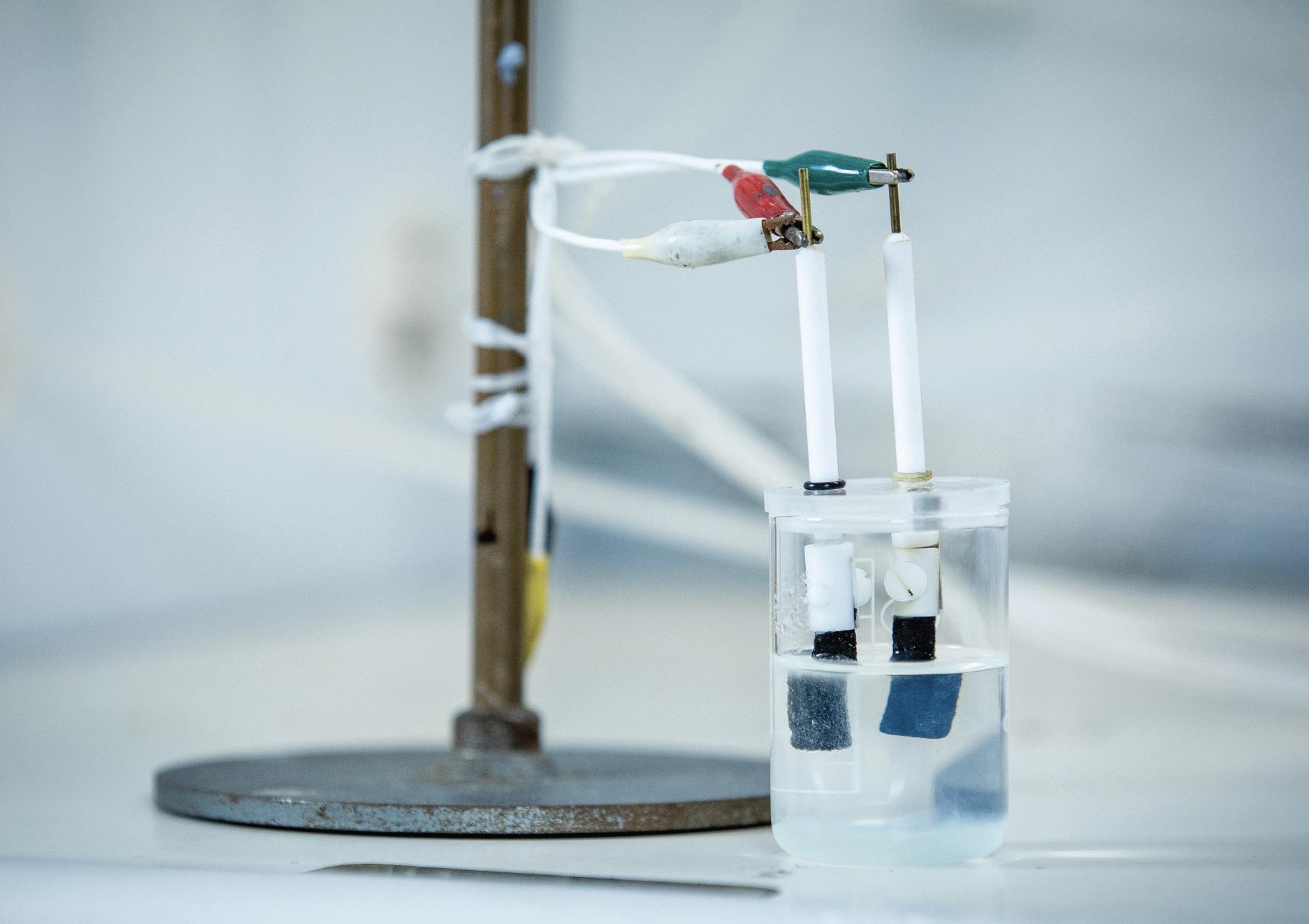 Figure 1. The experimental set-up used to evaluate the catalyst that effectively splits sea water into hydrogen and oxygen is shown. Figure courtesy of RMIT University.
Figure 1. The experimental set-up used to evaluate the catalyst that effectively splits sea water into hydrogen and oxygen is shown. Figure courtesy of RMIT University.
The structure of the catalyst was able to eliminate any possibility of chloride interference including the minimization of the chloride evolution reaction. Mahmood says, “Nitrogen bonds with molybdenum and nickel decreased the diffusion rates of undesirable ions such as chloride anions. Establishment of polyanions such as phosphates, nitrates and hydroxyl ions under the alkaline conditions led to the formation of a protective coating on the catalyst that did not interfere with splitting of water.”
Testing of the catalyst has worked well on the lab scale. Mahmood says, “Our next step is to scale up the process so that we can work toward commercialization.” Additional information can be found in a recent article
2 or by contacting Mahmood at
nasir.mahmood@rmit.edu.au.
REFERENCES
1.
Canter, N. (2022), “Generation of hydrogen using aluminum nanoparticles,” TLT,
78 (12), pp. 14-15. Available
here.
2.
Loomba, S., Khan, M., Haris, M., Mousavi, S., Zavabeti, A., Xu, K., Tadich, A., Thomsen, L., McConville, C., Li, Y., Walia, S. and Mahmood, N. (2023), “Nitrogen-doped porous nickel molybdenum phosphide sheets for efficient seawater splitting,”
Small, https://doi.org/10.1002/smll.202207310.