Curing tribological ills with ILs
By Drs. Wilfred T. Tysoe & Nicholas D. Spencer, Contributing Editors | TLT Cutting Edge June 2023
A combination of carefully controlled tribological experiments and X-ray photoelectron spectroscopic analyses sheds light on the lubrication mechanisms of ionic liquids.

Ionic liquids (ILs) are remarkable fluids that consist of organic molecules that are either charged negatively (anions) or positively (cations) so that electrostatic forces keep them together, much in the same way as inorganic salts, such as sodium or potassium chloride. Because the organic fragments in ILs that surround the charged centers are much larger than in inorganic salts, the interactions are much weaker, so they tend to form liquids rather than solids. For the same reason, van der Waals’ forces also contribute significantly to their intermolecular interactions. Consequently, they have exceedingly low vapor pressures and are therefore ideal candidates as space lubricants. There are now many thousand different types of ionic liquids with different ion combinations that display a wide range of tunable properties and potential applications, notably as lubricants, and in particular for use in electric vehicles.
However, despite their potential utility as lubricants, and perhaps because of their chemical complexity, little is known about how they function as lubricants. This has recently been remedied by STLE member professor Filippo Mangolini from the Texas Materials Institute and the Walker Department of Mechanical Engineering at the University of Texas at Austin, working with students Jieming Yan and Hsu-Ming Lien.
They carried out careful rubbing experiments on the chlorine-free ionic liquids depicted in Figure 1(a), slightly varying the anionic and cationic structures, with the cations differing in the symmetry and length of the alkyl chains attached to the amide center. Chlorine-containing ILs were avoided because they corrode the surface, and [HMIM][TFSI] (1-Hexyl-3-methylimidazolium bis(trifluormethylsulfonyl)imide) was investigated as a control because its properties have been very well documented. Tribological tests were carried out for ball-onflat sliding at ~350 K, and the samples were then scrupulously cleaned for analysis by X-ray photoelectron spectroscopy (XPS).
Figures 1(b) and (c) show histograms of the limiting, steady-state friction and wear behavior, which are influenced by the nature of the side groups in the ionic liquids. However, the friction and wear properties were not correlated.
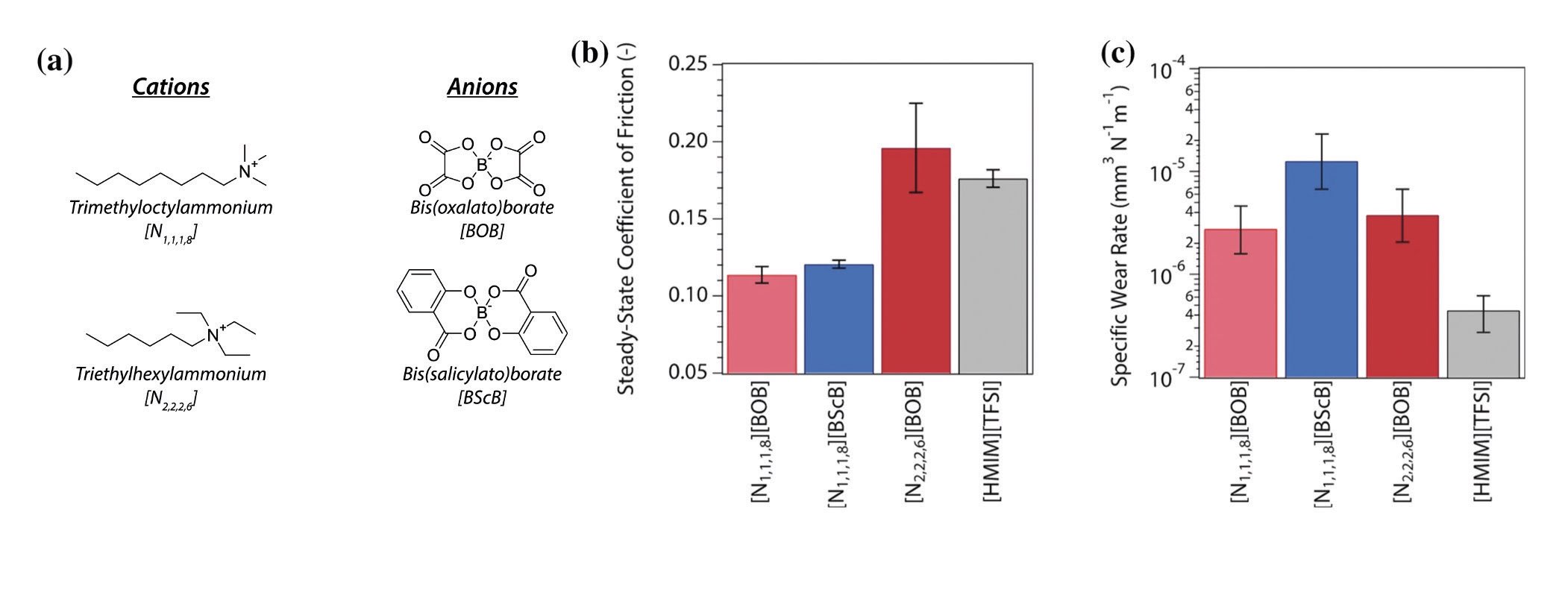 Figure 1. (a) Depiction of the tetraalkylammonium cations and orthoborate anions used in Ref. 1 and their shorthand notations, (b) bar graph of the final values of the coefficients of friction obtained during reciprocating ball-on-disc (52100 vs. 52100 steel) tests carried out at 353 ± 3 K in neat [N1,1,1,8][BOB], [N1,1,1,8][BScB], [N2,2,2,6][BOB], and [HMIM][TFSI] and (c) bar graph of the corresponding values of the wear rates. Figure published with permission from Ref. 1.
Figure 1. (a) Depiction of the tetraalkylammonium cations and orthoborate anions used in Ref. 1 and their shorthand notations, (b) bar graph of the final values of the coefficients of friction obtained during reciprocating ball-on-disc (52100 vs. 52100 steel) tests carried out at 353 ± 3 K in neat [N1,1,1,8][BOB], [N1,1,1,8][BScB], [N2,2,2,6][BOB], and [HMIM][TFSI] and (c) bar graph of the corresponding values of the wear rates. Figure published with permission from Ref. 1.
The researchers then investigated the origins of the differences by using XPS to analyze the surfaces after rubbing. An advantage of ionic liquids for this purpose is that their vapor pressures are generally low enough that they can withstand the ultrahigh vacuum conditions required to collect XPS data. This allowed detailed comparisons to be made with the changes that had occurred on the rubbed regions. They detected no ILs on the parts of the sample that had not been rubbed, strongly suggesting that the traces in the rubbed region were due to IL reaction products.
The resulting proposed reaction pathways are depicted graphically in Figure 2 for low- ([N
1,1,1,8][BOB] or [N
1,1,1,8][BScB]) and high-friction ([N
2,2,2,6][BScB]) ionic liquids. The low-friction ILs form solid-like layers on the surface because of the strong intermolecular interactions between longer alkyl chains. Here the forces are low enough not to induce any mechanochemical decomposition.
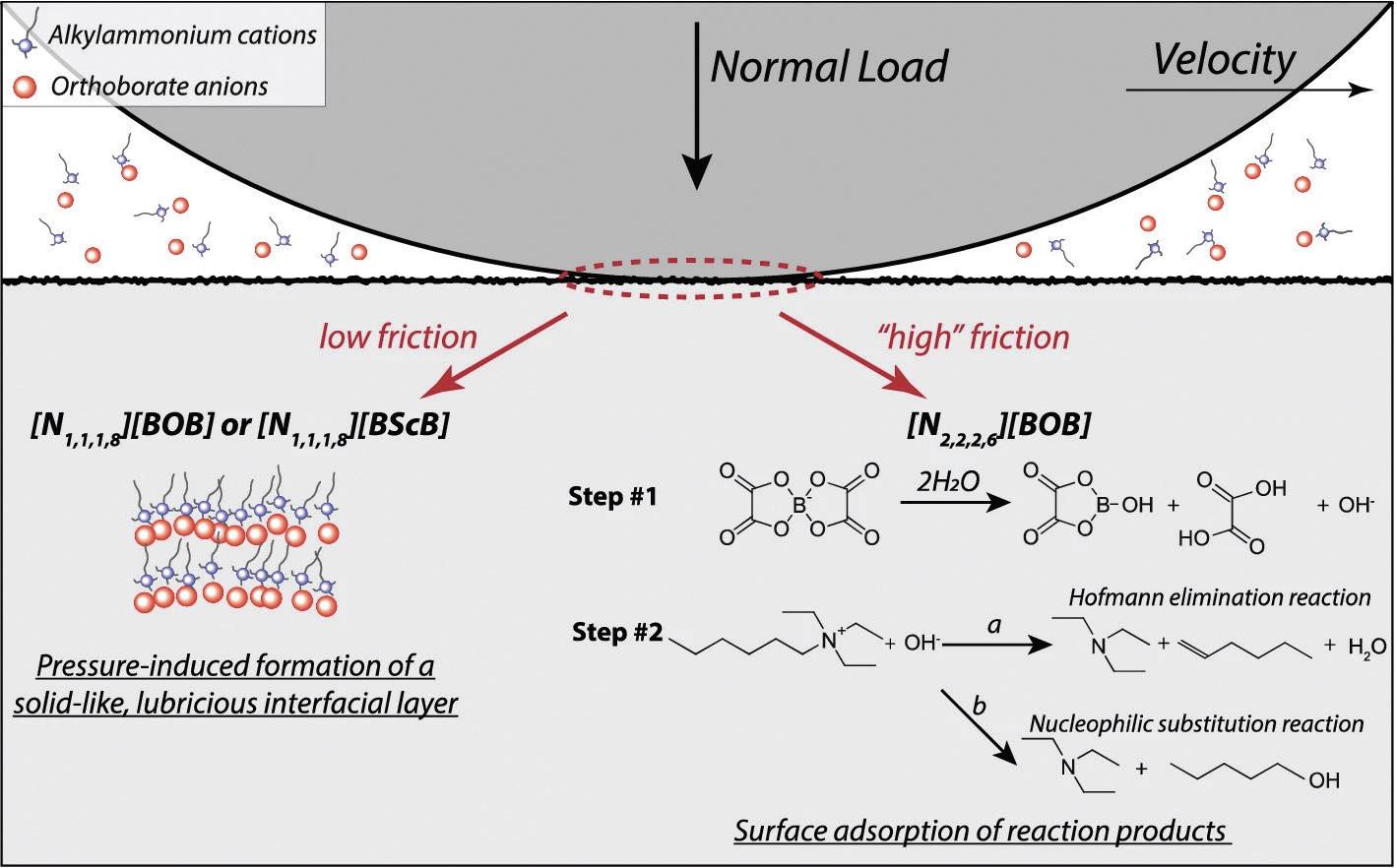 Figure 2. Depiction of the model used to describe the friction response of steel/steel contacts in the presence of tetraalkylammonium orthoborate ILs. Figure published with permission from Ref. 1.
Figure 2. Depiction of the model used to describe the friction response of steel/steel contacts in the presence of tetraalkylammonium orthoborate ILs. Figure published with permission from Ref. 1.
The weaker, van der Waals interactions between ILs with shorter-chain, more symmetric alkyl groups prevent the formation of the compact, low-friction layers to induce mechanochemical decomposition of the ionic liquids. Based on the XPS analyses, this is proposed to be initiated by B–O bond scission to produce trivalent borate esters, oxalic acid and hydroxide ions
(Step #1 in Figure 2). The resulting hydroxides facilitate the degradation of alkylammonium cations through either Hoffmann elimination or nucleophilic substitution reactions
(Step #2 in Figure 2).
This work shows how well-controlled tribological experiments combined with detailed surface analyses can lead to an understanding of the surface processes that control the friction, wear and reactivity of ionic liquids. This will hopefully lead to the development of design principles for synthesizing such chemically complex but versatile lubricants.
REFERENCE
1.
Yan, J., Lien, H-M. and Mangolini, F. (2023), “Linking Molecular Structure and Lubrication Mechanisms in Tetraalkylammonium Orthoborate Ionic Liquids,”
Tribology Letters, 71, 41.
Eddy Tysoe is a distinguished professor of physical chemistry at the University of Wisconsin-Milwaukee. You can reach him at wtt@uwm.edu.
Nic Spencer is emeritus professor of surface science and technology at the ETH Zurich, Switzerland, and editor-in-chief of STLE-affiliated Tribology Letters journal. You can reach him at nspencer@ethz.ch.