HIGHLIGHTS
•
Modification of lithium metal anodes used in batteries was accomplished through the brushing of phosphorus pentasulfide powder on the electrode surface.
•
Evaluation testing showed that a new substance, lithium phosphide sulfide, forms immediately when phosphorus pentasulfide is applied to modify the surface of the anode.
•
Electrochemical performance testing showed that anodes treated with phosphorus pentasulfide retained 70% more capacity than those which are untreated after 340 charge-discharge cycles.
Research is ongoing to determine how to maximize the operating life of batteries. The demanding operating conditions in applications such as electric vehicles are making it challenging to overcome issues that are limiting battery life and even leading to the possibility of short circuits that can cause catastrophic failures.
One issue that has emerged in a number of different battery types including lithium metal is the growth of needle-like structures known as dendrites. Once formed at a specific electrode, dendrites can grow from one electrode to another leading to a battery short circuit.
In a previous TLT article,
1 researchers studied the ion transport dynamics near the cathode of a battery using microfluidics in order to better understand the growth rate of dendrites. By increasing the cross-flow rate of ions, dendrite formation decreased by as much as 98%. At high ion flow rates, the growth of dendrite precursors known as vortexes on the surface of electrodes drops significantly leading to a smoother electrode surface.
One of the leading battery types under evaluation is lithium-ion, which uses graphite as the anode. The high weight of graphite has led researchers to switch to using lithium metal as the anode, which can produce a 10-fold reduction in weight, which is an important issue in applications such as electric vehicles.
The high reactivity of lithium leads to the immediate production of a solid electrolyte interphase (SEI) as a battery starts cycling. But SEI instability can limit battery performance. Weiyin Chen, graduate student in the chemistry department at Rice University in Houston, Texas, says, “Past approaches to stabilize lithium metal anodes involved the application of powders in solutions in an effort to passivate the electrode surface. The problem has been finding a solvent that is compatible with powders due to differences in polarity. Non-polar powders are not compatible with polar solvents and vice versa.”
A new technique for modification of lithium metal anodes has been developed that eliminates the use of a solvent and enables powders to be directly applied to the electrode surface.
Phosphorus pentasulfide modified anodes
Chen and James Tour, T.T. and W.F. Chao professor of chemistry and professor of materials science and engineering at Rice University, and their colleagues are now reporting that brushing lithium anodes with specific powders will modify the surface of a lithium metal anode to minimize dendrite formation and significantly increase their operating life. Initially work was conducted with the non-polar powders: polytetrafluoroethylene, phosphorus pentasulfide, molybdenum disulfide and the polar polymer, polyacrylonitrile.
A schematic of the brushing procedure is shown in Figure 2. Chen says, “Due to the reactivity of lithium, the brushing is done in a glove box under inert conditions. Initially, the metal surface is brushed gently until it is matted to remove the presence of lithium oxide. Then the powder is applied across the surface of the lithium in a monodirectional manner. The powder is rubbed into the lithium in a manner similar to finger painting on paper. Excess powder is removed, and the process is repeated twice.”
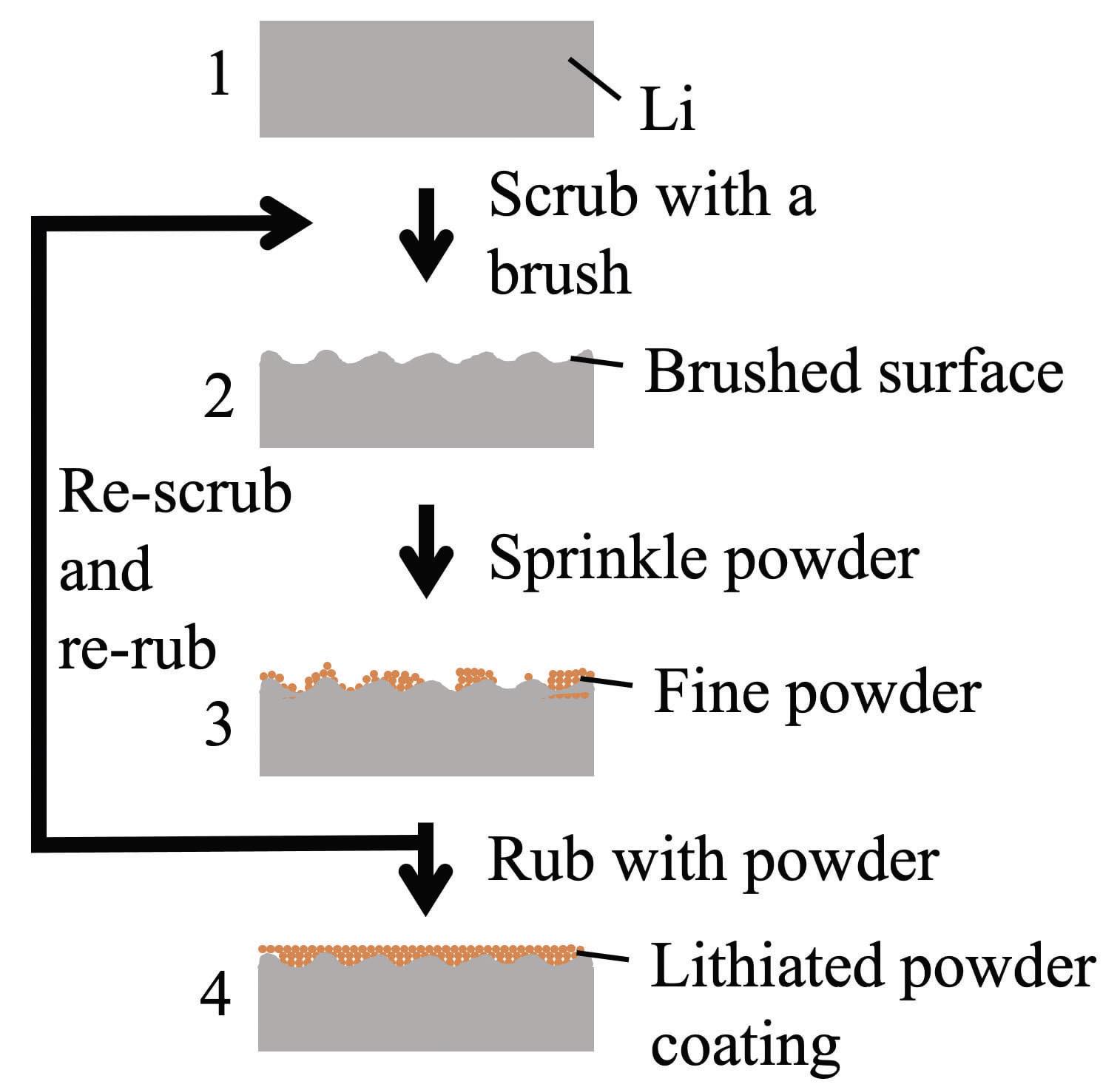 Figure 2. The procedure for modifying a lithium metal anode with phosphorus pentasulfide is shown. Superior performance was found when the modified anode was evaluated. Figure courtesy of Rice University.
Figure 2. The procedure for modifying a lithium metal anode with phosphorus pentasulfide is shown. Superior performance was found when the modified anode was evaluated. Figure courtesy of Rice University.
The net effect is to produce a homogeneous layer of powder on the lithium surface. Chen says, “We found the most success with phosphorus pentasulfide, which reacts almost instantaneously with lithium under these conditions to modify the surface of the anode. Raman spectroscopy identified the substance formed on the surface to be a lithium phosphide sulfide.”
Electrochemical performance testing was done in coin cells that contained both phosphorus pentasulfide modified lithium and bare lithium anodes combined with lithium-iron-phosphate-oxide cathodes. Results showed that batteries containing powder treated lithium anodes retained 70% more capacity than those which are untreated after 340 charge-discharge cycles.
Further testing showed that the phosphorus pentasulfide modified anodes exhibit and sustain ultralow polarization for great er than 4,000 hours, which is eight times longer than for bare lithium anodes. Chen says, “This result indicates that the uniform electrodeposition of the phosphorus pentasulfide stabilizes the electrode leading to superior performance.”
The powder has a thickness between 2-10 microns. Tour says, “The surface coating produced during the interaction of phosphorus pentasulfide and lithium exhibits good ionic conductivity. The coating effectively tunes the surface energy of the electrode leading to more uniform behavior and eliminates the possibility of dendrite formation. Lithium metal is not lost, and the coating also facilitates the movement of lithium ions to the cathode.”
The researchers further demonstrated the use of this concept in brushing phosphorus pentasulfide onto a sodium electrode. Chen says, “Similar testing was done, and the phosphorus pentasulfide modified electrode displays a lower steady overpotential.”
Tour says, “The brushing procedure has the potential to be commercialized because it is straight forward and easily mechanized.”
Future work will involve evaluation of brushed electrodes using solid electrolytes, which are now finding favor within the battery industry. Additional information on this research can be found in a recent paper
2 or by contacting Tour at
tour@rice.edu.
REFERENCES
1.
Canter, N. (2021), “Inhibition of dendrite formation: Ion flow,” TLT,
77 (6), pp. 32-33. Available
here.
2.
Chen, W., Salvatierra, R., Li, J., Luong, D., Beckham, J., Li, V., La, N., Xu, J. and Tour, J. (2022), “Brushed metals for rechargeable metal batteries,”
Advanced Materials, 34 (31), 2202668.