As progress is made to convert to a more sustainable, hydrogen-based economy, efforts are underway to find a cost-effective process for the manufacture of green hydrogen. The challenge for researchers is to find an effective catalyst to split water.
In a recent TLT article,
1 researchers determined that hydrogen intercalation can be used to facilitate the production of hydrogen. When hydrogen was inserted into tungsten trioxide, the metal oxide was converted into an electronic conductor through the formation of hydrogen-oxygen bonds in the catalyst and the reduction of the metal atom’s oxidation state. Hydrogen formation occurs once a water mediated reaction produces a hydroxyl group allowing hydrogen atoms on adjacent tungsten atoms to combine forming molecular hydrogen.
Aluminum has emerged as an attractive catalyst for producing hydrogen but has limitations. This metal can theoretically react with water to produce hydrogen and aluminum oxide. The best strategy for generating hydrogen through this process is to work with aluminum nanoparticles, but their synthesis is very difficult to achieve. Efforts to use aluminum derivatives such as lithium aluminum hydride, alane, triisobutylaluminum and diissobutylaluminum hydride have not been successful. These aluminum hydride derivatives are expensive and difficult to work with because they are explosive.
The other issue with working with aluminum is finding a procedure for minimizing the formation of an oxide coating on the metal. Aluminum readily reacts with oxygen at room temperature, and the presence of aluminum oxide inhibits reaction with water.
A promising approach for producing aluminum nanoparticles is to produce composites through reaction with liquid metal alloys such as one prepared from gallium, indium and tin. The reaction of aluminum and gallium with water has been known since the 1970s, and videos of it are easy to find online. Some success was found in generating hydrogen, but the reaction conditions were not suitable, and the yield was poor.
Bakthan Singaram, professor of chemistry and biochemistry at the University of California Santa Cruz in Santa Cruz, Calif., says, “The study grew out of a conversation he had with one of our students who had seen some videos and started experimenting with aluminum-gallium hydrogen generation in his home kitchen. This initial work led us to initiate a project to determine how to work with these two elements to maximize production of hydrogen.”
Gallium-aluminum composite
Singaram and Scott Oliver, professors of chemistry at the University of California Santa Cruz, and their colleagues explored the interaction of gallium with aluminum and found that using the right stoichiometry can produce a 90% yield of hydrogen at ambient temperature. The researchers are able to take advantage of gallium’s liquid state (melting point 29.9 C) by adding it to aluminum foil and then pressing the gallium into the aluminum for 10 minutes. The result is the formation of a gallium-aluminum composite. Addition of the composite to a flask followed by the introduction of deionized water led immediately to the evolution of hydrogen gas. Under small scale conditions, where 10 milliliters of water are used, the reaction was completed in approximately 15 minutes.
Singaram says, “We found that the optimum atomic ratio of gallium to aluminum is 3:1. The higher concentration of gallium is needed to dissolve the oxide coating from the surface of the aluminum, facilitate the generation of nanoparticles and prevent them from agglomerating. The gallium also serves to prevent oxidation of the aluminum surface leaving the metal in position to react with water.”
In the mechanism for this process, Singaram speculates that aluminum acts as both a Lewis acid and a Lewis base in reacting with water. He says, “As a Lewis acid, aluminum coordinates with an oxygen on the water molecule. From the Lewis base standpoint, aluminum will react with a polarized hydrogen atom. The next step is the simultaneous breaking of an oxygen-hydrogen bond and formation of an aluminum-hydrogen bond leading to the formation of hydrogen gas and aluminum oxide.”
The researchers then examined the reactivity of different aluminum sources such as commercial beverage cans, food receptacles and soda cans. In all cases, a high yield of hydrogen was obtained with all of these sources.
A similar approach was taken with trying different sources of water including tap water, rain water, recycled water and ocean water. Oliver says, “We evaluated water with different levels of electrolytes and found the reaction will work effectively in all cases except for ocean water. Our hypothesis is that the chloride present in sea water interferes with the process by reacting with the aluminum. The pH of the water also is not a factor, and we found the reaction works well when water is at a neutral pH.”
Figure 1 shows that the hydrogen generation process is sustainable. The researchers are able to recycle gallium and use it multiple times without a loss of reactivity. Aluminum oxide also can be converted back to aluminum for further use.
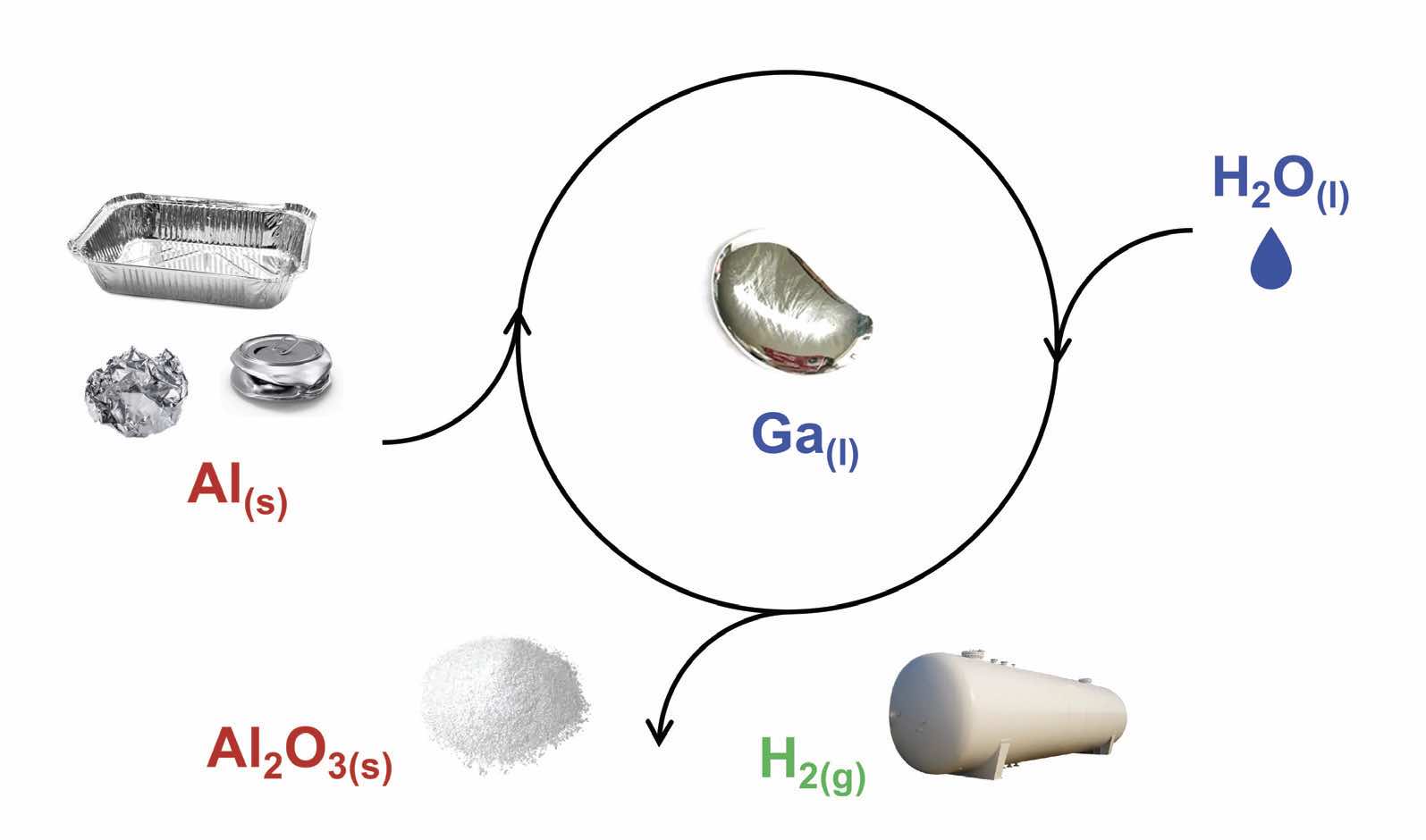 Figure 1. The process for reacting aluminum (Al) with water (H2O) in the presence of gallium (Ga) is shown. Hydrogen (H2) and aluminum oxide (Al2O3) are the products. Sustainability is achieved through recycling the gallium for use multiple times and converting aluminum oxide back to aluminum for further use. Figure courtesy of the University of California Santa Cruz.
Figure 1. The process for reacting aluminum (Al) with water (H2O) in the presence of gallium (Ga) is shown. Hydrogen (H2) and aluminum oxide (Al2O3) are the products. Sustainability is achieved through recycling the gallium for use multiple times and converting aluminum oxide back to aluminum for further use. Figure courtesy of the University of California Santa Cruz.
The researchers demonstrated the utility of the process by using hydrogen generated by the reaction of the gallium-aluminum composite in a hydrogenation reaction with the alkene, 4-phenyl-1-buten-4-ol. Conversion in high yield to the corresponding alkane was found at high yield after one hour of mixing at room temperature.
The use of the gallium-aluminum composite appears to be a promising way to produce green hydrogen in high yield under mild conditions. Further research will be done to increase the hydrogen yield when using sea water.
Additional information can be found in a recent paper
2 or by contacting Singaram at
singaram@ucsc.edu and Oliver at
soliver@ucsc.edu.
REFERENCES
1.
Canter, N. (2022), “Potential for metal oxide catalysts to facilitate sustainable processes,” TLT,
78 (8), pp. 16-17. Available
here.
2.
Amberchan, G., Lopez, I., Ehlke, B., Barnett, J., Bao, N., Allen, A., Singaram, B. and Oliver, S. (2022), “Aluminum nanoparticles from a Ga-Al composite for water splitting and hydrogen generation,”
Applied Nano Materials, 5 (2), pp. 2636-2643.