HIGHLIGHTS
•
Hydrogen intercalation is an insertion process that places hydrogen ions and electrons in a metal oxide lattice.
•
Intercalation enhances the performance of the catalyst, tungsten trioxide by converting this metal oxide into an electronic conductor.
•
The proposed mechanism first involves a tungsten atom absorbing a hydrogen ion, followed by the formation of a hydroxyl group on an adjacent tungsten atom through a water mediated reaction. The two hydrogen atoms then link up to each other forming molecular hydrogen.
Research is continuing to determine how to effectively produce green hydrogen in a sustainable manner through the splitting of water. Much of the attention has been paid to the oxygen evolution reaction, which is a four-electron transfer process.
In a previous TLT article,
1 a study was conducted that showed a change in catalyst structure leads to improved performance in the oxygen evolution reaction. Specific perovskite oxide catalysts containing rare earth and transition metals were found to undergo a structural change known as surface reconstruction. Under the acidic conditions of the oxygen evolution reaction, specific metal atoms leached out of the perovskite oxide catalyst facilitating the formation of an amorphous layer on the surface of the material. Catalyst activity increased by two orders of magnitude.
The other reaction in splitting water is the hydrogen evolution reaction. While this process is not as challenging, there is still a need for finding catalysts that can enhance hydrogen formation.
One approach is to examine catalysts that can place protons and electrons into a metal oxide lattice. This is known as hydrogen intercalation. Dr. James McKone, assistant professor of chemical engineering in the Swanson School of Engineering at the University of Pittsburgh in Pittsburgh, Pa., says, “Hydrogen intercalation is an insertion process by which a host absorbs hydrogen ions in an electrochemical process. This is similar to what is occurring in an electric vehicle battery where the species undergoing intercalation is typically lithium.”
Intercalation can be done with any charged species according to McKone. One potential benefit of this process is to determine if intercalation can alter the structure of a metal oxide catalyst to improve performance.
McKone says, “The potential benefit for hydrogen intercalation is to determine if it can positively impact the bulk structure of a catalyst. While catalysis is still occurring on the surface, changes in the interior structure of the catalyst may lead to an improvement in the conversion rate of the hydrogen evolution reaction.”
McKone points out that metal oxide catalysts that undergo hydrogen intercalation are reduced leading to the formation of metal oxide hydrogen bronze. He says, “This electrochemical process generates a change in the appearance of the color of the metal oxide catalyst. Metal oxides are typically white or gray in appearance prior to hydrogen intercalation. Upon the introduction of hydrogen ions, metal oxides are converted into brown, blue or black species. After further hydrogen reduction, the metal oxides assume a lustrous metallic appearance, which is why they are called bronzes.”
A new computational-led study with experimental validation has now been reported showing that hydrogen intercalation is integral to the mechanism by which some metal oxides perform the hydrogen evolution reaction. The computational study was led by Dr. Giannis Mpourmpakis, associate professor of chemical engineering and Bicentennial Alumni Faculty Fellow at the University of Pittsburgh, and the experimental validation was done in McKone’s lab. Evan Miu, a doctoral researcher and National Science Foundation graduate fellow, performed the computational and experimental work in this study under the mentorship of Mpourmpakis and McKone.
Tungsten trioxide
McKone, Mpourmpakis and Miu demonstrated the potential of hydrogen intercalation in affecting catalyst performance by working with tungsten trioxide. They say, “We have been working with tungsten trioxide for some time and are very familiar with its catalytic properties. In earlier work, we studied the way hydrogen intercalated into tungsten trioxide under various conditions. This study extended that work to see how this type of behavior also impacts its catalytic behavior.”
A combination of computational modeling and experiments was done to assess how tungsten trioxide’s reactivity changes when exposed to hydrogen. McKone says, “This type of catalysis can be considered as a combination of acid base reactions and redox reactions. The intercalation process inserts hydrogen ions into the crystalline structure of the metal oxide along with an equal number of electrons. This transforms the metal oxide into an electronic conductor.”
Two factors align to change the reactivity of the tungsten trioxide. McKone says, “Our analysis shows that the hydrogen ions form bonds with oxygen atoms inside the metal oxide structure. This is combined with a change in the oxidation state of the metal. Tungsten atoms undergo a reduction in the presence of electrons, which leads to this metal atom’s oxidation state changing from (+6 in tungsten trioxide) to +5 or possibly +4.”
The researchers applied quantum chemical, density functional theory (DFT) calculations to better understand how tungsten trioxide interacts with hydrogen ions. Mpourmpakis says, “To be able to accurately simulate catalytic behavior of metal oxides, such as tungsten trioxide, it is essential to perform quantum mechanical calculations. In addition, to make predictions that connect the molecular level information of these calculations with the experimentally observed catalytic behavior, we need to use advanced computational methods such as constant potential DFT and apply multiscale modeling. In this way, we can simulate how many electrons flow during electrocatalytic reaction events and connect with the experimentally measured hydrogen evolution behavior.”
Mpourmpakis continues, “We have been working for more than a decade now on understanding acid base chemistries and identifying metal oxides for industrial applications. This collaborative study moves away from energy intense chemical processes toward a focus on utilizing renewable electricity to produce hydrogen with a catalyst that acts as a sponge to accept and give hydrogen.”
Data obtained by the researchers was used to determine the mechanism for how tungsten trioxide converted hydrogen ions into hydrogen gas. McKone says, “The classic mechanism for how the best metal catalysts (based on precious metals; platinum, palladium and rhodium) function is to bind hydrogen ions on the surface to adjacent metal atoms and then bond two of them together. But in the case of tungsten trioxide, this is not possible because two tungsten atoms are not too far away from one another.”
The proposed mechanism starts with a hydrogen ion absorbing onto a tungsten atom at the surface. A hydroxyl group on an adjacent tungsten atom is formed through a water mediated reaction setting the stage for a second hydrogen ion to bond to the initial hydrogen linked to the tungsten atom. Desorption of the hydrogen molecule ensues leading to the completion of the reaction and preparing the catalytic process to start again.
Figure 3 shows the interactions among hydrogen ions, hydroxyl groups, water and tungsten trioxide.
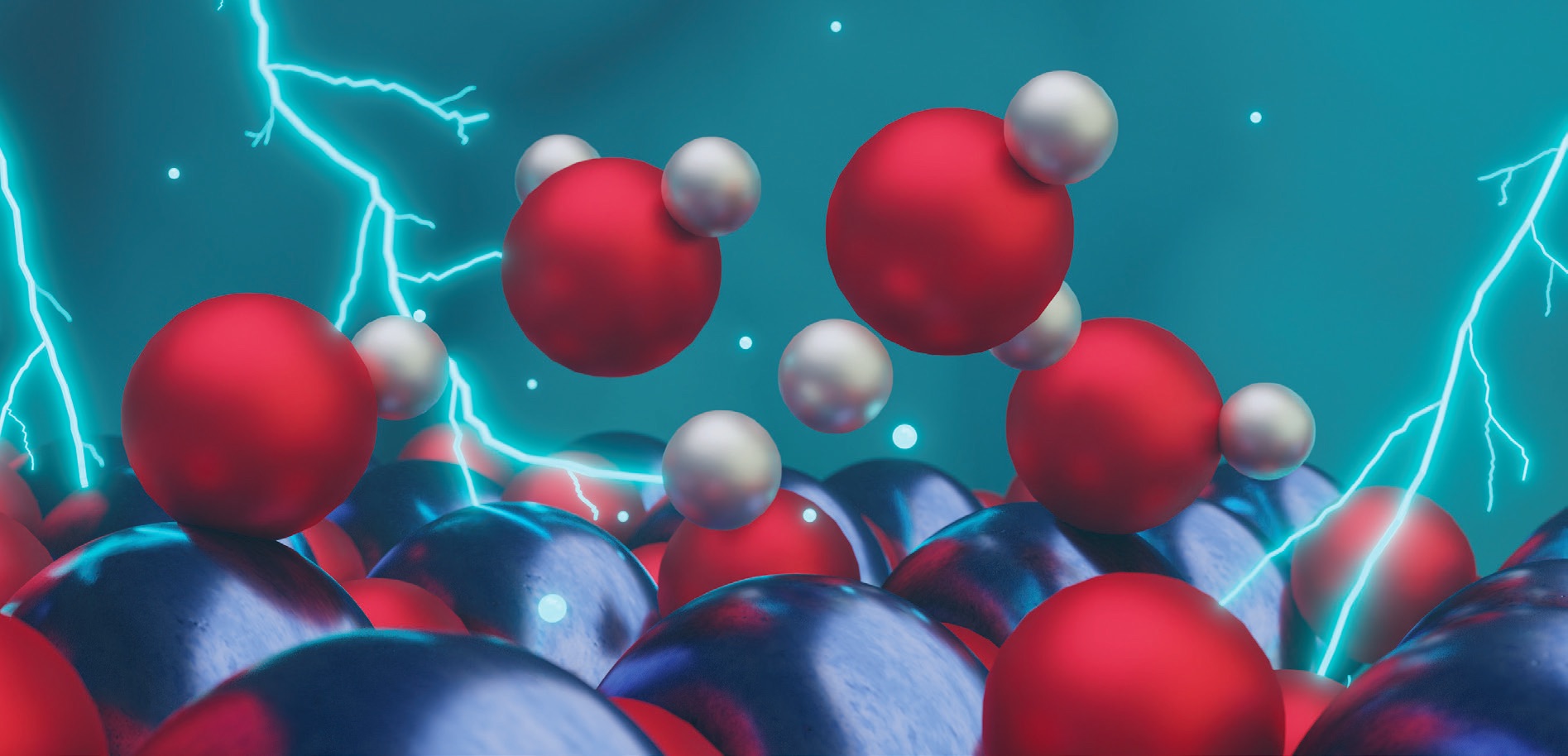 Figure 3. The interaction of tungsten trioxide with hydrogen atoms, hydroxyl groups and water is shown. Tungsten atoms are in blue, hydrogen atoms are in gray and oxygen atoms are in red. Figure courtesy of the University of Pittsburgh.
Figure 3. The interaction of tungsten trioxide with hydrogen atoms, hydroxyl groups and water is shown. Tungsten atoms are in blue, hydrogen atoms are in gray and oxygen atoms are in red. Figure courtesy of the University of Pittsburgh.
McKone says, “Our interest in working with tungsten trioxide was to show that hydrogen intercalation is important in allowing metal oxides to produce hydrogen. But tungsten trioxide does not exhibit sufficient efficiency compared to precious metals even though it is lower in cost.”
The work done demonstrates the potential for evaluating the performance of other metal oxide catalysts in other catalytic processes using intercalation. The team is very optimistic that hydrogen can be generated in this fashion to be used in reacting with carbon dioxide in a sustainable manner. The hydrogen could be used, for example, to make synthesis gas for the formation of hydrocarbons including lubricant base oils.
Additional information on this research can be found in a recent article
2 or by contacting McKone at
jmckone@pitt.edu and Mpourmpakis at
gmpourmp@pitt.edu.
REFERENCES
1.
Canter, N. (2022), “Metal leaching and surface manipulation of water oxidation catalysts,” TLT, 78 (3), pp. 18-19. Available
here.
2.
Miu, E., McKone, J. and Mpourmpakis, G. (2022), “The sensitivity of metal oxide electrocatalysis to bulk hydrogen intercalation: Hydrogen evolution on tungsten oxide,”
Journal of the American Chemical Society, 144 (14), pp. 6420-6433.