KEY CONCEPTS
•
A catalyst based on iron and known by its chemical symbol designation, Fe-N-C, is under evaluation in fuel cells because it is cheaper than the traditional platinum-based materials.
•
Tantalum-titanium dioxide radical scavengers have been found to improve the performance of the Fe-N-C catalyst by minimizing the presence of hydroxyl and hydroperoxyl radicals and hydrogen peroxide.
•
Use of tantalum and titanium at a ratio of 6:4 provides the highest chemical stability and best performance for the radical scavenger.
Fuel cells remain a feasible approach for use as an efficient energy conversion device that is sustainable. The fundamental idea of obtaining energy from catalyzing the reaction of hydrogen with oxygen using a proton exchange membrane is appealing, but there continue to be technical challenges that need to be overcome.
In a previous TLT article,
1 researchers developed an ionomeric binder material that was placed on a phosphoric acid-doped polybenzimidazole membrane for use in high-temperature membrane fuel cells designed to operate at temperatures above 100 C. High-temperature fuel cell membranes have potential to be used in place of conventional proton exchange membranes because of their simpler design and ability to use lower purity hydrogen. The binder material enabled the high-temperature fuel cell to display much higher performance and better tolerance of water.
The major catalysts used in the oxygen reduction reaction that fuel cells undergo are based on platinum-based materials. Due to their high cost, researchers have been evaluating platinum group metal-free (PGM-free) catalysts based on a transition metal, nitrogen and carbon. One type that is under evaluation is based on iron and is known by its chemical symbol designation as Fe-N-C catalysts.
Reza Shahbazian-Yassar, professor of mechanical and industrial engineering at the University of Illinois Chicago’s college of engineering in Chicago, says, “Interest in evaluation of Fe-N-C catalysts is due to all of the elements being readily available and lower in cost than platinum group metals. The problem with these catalysts is they quickly lose performance over the first 100 hours of fuel cell operation. They cannot cycle in the same manner as platinum catalysts because of their instability in the high acidic environment of proton exchange membrane fuel cells.”
This loss of performance is due to catalyst degradation. Shahbazian-Yassar says, “Hydroxyl and hydroperoxy radicals present due to the formation of hydrogen peroxide, a product of incomplete oxygen reduction, can reduce the efficiency of Fe-N-C catalysts through two pathways. The first involves oxidation of carbon to produce carbon dioxide and the second is the formation of oxygen functional groups on the surface of the catalyst. In both cases, damage to the catalyst facilitates further production of hydrogen peroxide, which then increases the concentration of these catalyst damaging radicals.”
A new approach for protecting the Fe-N-C catalysts by minimizing the presence of hydrogen peroxide in the fuel cell has now been developed.
Radical scavengers
A team of researchers from multiple institutes determined that introducing radical scavengers to the Fe-N-C catalyst provides a good opportunity for improving performance. The purpose of these radical scavengers is to chemically react with hydroxyl and hydroperoxyl radicals and hydrogen peroxide to minimize their presence near the catalyst.
To achieve this objective, the researchers decided to evaluate a radical scavenger based on tantalum and titanium dioxide. Shahbazian-Yassar says, “Computational analysis showed that this scavenger present as nanoparticles in close proximity to the Fe-N-C catalyst should be effective.”
Uniformly dispersed radical scavenger particles, with diameters less than 10 nanometers, were produced using a high-temperature pulse approach from titanium (IV) isopropoxide and tantalum (V) ethoxide. The researchers synthesized radical scavengers using different atomic ratios of tantalum to titanium. Shahbazian-Yassar says, “Our work focused on characterizing the nanoparticles having a tantalum to titanium ratio of 6:4 because that stoichiometry produced the best results in the electrochemical studies.”
The radical scavengers were evaluated by incorporating them into a Fe-N-C catalyst prepared from a zeolitic imidazolate framework. This PGM-free catalyst contains atomically dispersed active sites. Figure 2 shows how the tantalum-titanium dioxide radical scavengers can be dispersed through the Fe-N-C catalyst. A scanning transmission electron microscopy-energy dispersive spectroscopy (STEM-EDS) image of the scavenger embedded in the Fe-N-C catalyst is on the left and images of the individual elements (carbon: C, oxygen: O, tantalum: Ta and titanium: Ti) are on the right.
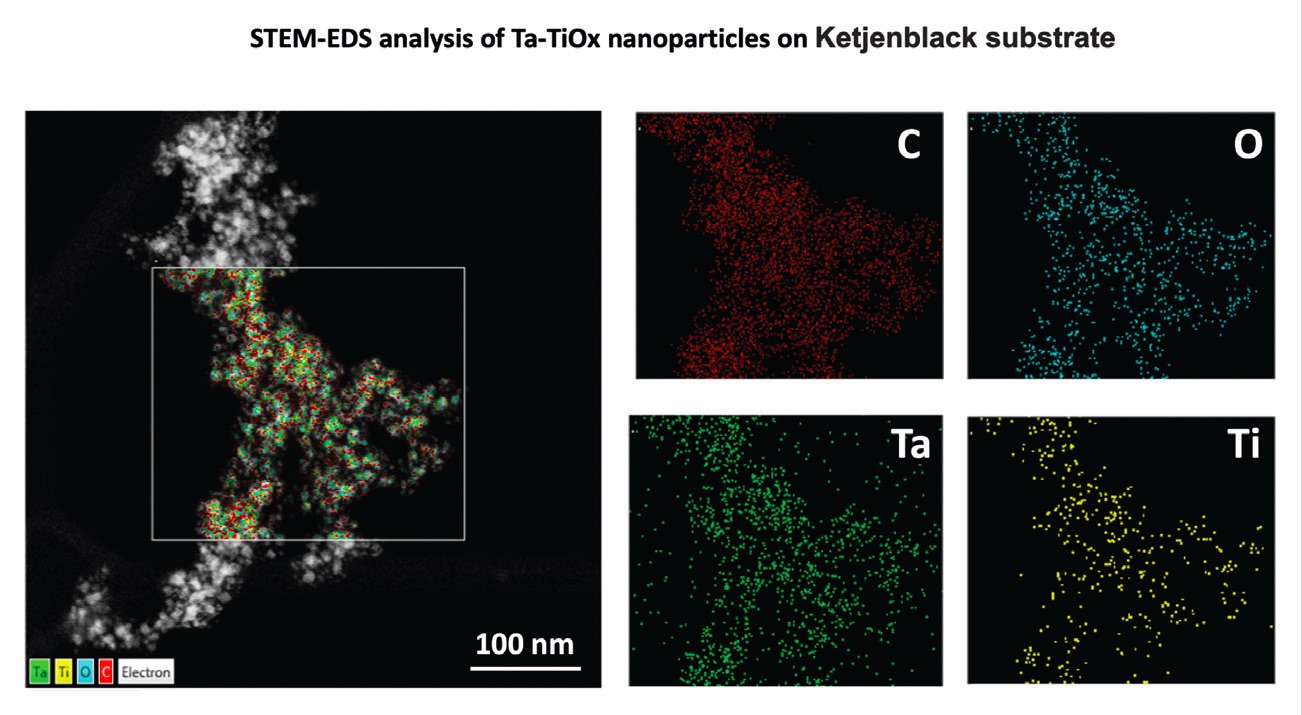 Figure 2. Scanning transmission electron microscopy-energy dispersive spectroscopy (STEM-EDS) images show how the radical scavenger is incorporated into the Fe-N-C catalyst on the left and shows the individual elements (carbon: C, oxygen: O, tantalum: Ta and titanium: Ti) on the right. Figure courtesy of the University of Illinois Chicago.
Figure 2. Scanning transmission electron microscopy-energy dispersive spectroscopy (STEM-EDS) images show how the radical scavenger is incorporated into the Fe-N-C catalyst on the left and shows the individual elements (carbon: C, oxygen: O, tantalum: Ta and titanium: Ti) on the right. Figure courtesy of the University of Illinois Chicago.
An initial study of the effectiveness of the radical scavengers was conducted through the use of a dye based on a fluorescein derivative that is susceptible to radical degradation. Fluorescence analysis showed that the dye degraded to a much lower extent in the presence of the tantalum-titanium dioxide radical scavenger.
The electrocatalytic performance of the radical scavenger with the Fe-N-C catalyst was evaluated by mixing the two components in an ink placed on a rotating ring disk electrode. A much lower percentage of hydrogen peroxide was detected when the radical scavenger was blended with the Fe-N-C catalyst. The performance of the Fe-N-C catalyst also was found to be better after 10,000 cycles when using the radical scavenger, which is an indication that durability has improved.
Shahbazian-Yassar indicated that the researchers obtained three key findings from studying the atomic structure of the radical scavenger when added to the Fe-N-C catalyst. He says, “The 6:4 ratio of tantalum to titanium represents the highest chemical stability for the radical scavenger under the acidic pH conditions used in operating the fuel cell. The mixing of the radical scavenger at this atomic ratio with the Fe-N-C catalyst produces a solid solution that has a large degree of entropy. This greatly improves the stability of the catalyst. A second finding is the ratio of the two components used in the radical scavenger leads to the formation of a rutile phase that is metastable and highly reactive.”
Shahbazian-Yassar continues, “We also found that the size of the nanoparticles used in the scavenger is very important. If the diameter of the nanoparticles is larger, then the radical scavenger loses surface area and is not as effective. A smaller diameter produces nanoparticles that are not stable.”
The researchers intend to better understand the atomic structure and compositional variations in alternative radical scavengers in the future. Shahbazian-Yassar says, “One way is to add more elements, which will boost the entropy of the system and should make the radical scavenger more effective.”
Additional information on this research can be found in a recent article
2 or by contacting Shahbazian-Yassar at
rsyassar@uic.edu.
REFERENCES
1.
Canter, N. (2021), “High-temperature membrane fuel cells,” TLT,
77 (3), pp. 14-15. Available
here.
2.
Xie, H., Xie, X., Prabhakaran, V., Saha, S., Gonzalez-Lopez, L., Phakatkar, A., Hong, M., Wu, M., Shahbazian-Yassar, R., Ramani, V., Al-Sheikhly, M. Jiang, D., Shao, Y. and Hu, L. (2022), “Ta-TiO
x nanoparticles as radical scavengers to improve the durability of Fe-N-C oxygen reduction catalysts,”
Nature Energy, 7 (3), pp. 281-289.