The most effective component in three-way catalysts used in standard catalytic converters is rhodium.
A new study found that deactivation of the rhodium/alumina catalyst used in catalytic converters is due to the formation of rhodium aluminate under high-temperature oxidizing conditions.
Reduction of catalytic activity was found to occur through two pathways where rhodium nanoparticles sinter into larger particles or decompose into rhodium atoms that migrate into the alumina to form rhodium aluminate.
With the prospect of vehicles powered by internal combustion engines operating globally for the foreseeable future as the dominant means of transportation, research needs to continue to better understand the operation of catalytic converters. Since the 1940s, catalytic converters have been used in automobiles to minimize emissions of carbon monoxide, hydrocarbons and nitrogen oxides.
The standard catalytic converter is prepared with three-way catalysts that are based on precious metals such as palladium, platinum and rhodium. In a previous TLT article,
1 work was conducted to better understand how palladium and platinum convert hydrocarbon combustion products to carbon dioxide and water. Oxidation was modeled based on how it occurs in a diesel engine, and the researchers used the model reaction of propene with oxygen. Active sites with nanoparticle sizes ranging from 10 to 20 nanometers were found to be particularly effective because they changed shape during the oxidation reaction resulting in the formation of more active catalytic sites. This was not seen with smaller nanoparticles.
This past study did not evaluate the performance of the third precious metal used in catalytic converters, rhodium. Dr. Cheng-Han Li and Dr. Joerg Jinschek, in the department of material science and engineering at The Ohio State University in Columbus, Ohio, say, “Rhodium has been found to exhibit superior performance in three-way catalysts to palladium and platinum because this precious metal can reduce the activation barrier of three-way catalyst reaction more significantly. Another factor is that rhodium exhibits a higher Fermi level than either palladium or platinum allowing for better catalysis under the net oxidation conditions found in the internal combustion engine.”
Rhodium is currently positioned on the common catalyst support alumina, which is used because it displays a high surface area. Unfortunately, the rhodium/alumina catalyst has been found to consistently deactivate under high-temperature (> 900 C) oxidizing conditions. Vehicles do not frequently operate under these extreme conditions. But the fact that drivers are keeping their vehicles for longer time frames and increasing the number of miles vehicles cover during their operating lifetime, the chances of encountering extreme conditions increases.
The result is that the chance for the rhodium/alumina catalyst to deactivate during vehicle use is higher, which can mean that vehicle emissions may increase. Rhodium also is becoming more expensive because supply has not kept pace with rising demand.
Research has been devoted to gaining a better understanding for how the rhodium/alumina catalyst becomes deactivated. No direct evidence has been reported on the mechanism for catalyst deactivation until now.
Rhodium aluminate
Li and his colleague Jinschek, formerly with The Ohio State University and now professor at the National Centre for Nano Fabrication and Characterization (DTU Nanolab) at the Technical University of Denmark (DTU) in Lyngby, Denmark, in collaboration with researchers at Ford Motor Co. in Dearborn, Mich., used several analytical methods to report that the reason for the deactivation of the rhodium/alumina catalyst at high-temperature oxidizing conditions is due to the formation of rhodium aluminate. To identify rhodium aluminate, the researchers conducted a series of light-off tests to determine the activity of a standard three-way catalyst under a range of temperature conditions. Added to the catalyst was a mixture of simulated exhaust gases that are present in an engine emissions stream including carbon monoxide, hydrogen, nitric oxide, propene, propane, oxygen, water, carbon dioxide and nitrogen.
Li says, “Lights-off testing is a measurement of the conversion rates of simulated exhaust gases versus temperature when the temperature of the catalyst is ramped up from room temperature to approximately 300 C to ideally reach 100% conversion rate. The temperatures where 50% or 90% conversion rates are reached can be used as matrices to evaluate the performance of the three-way catalyst.”
Li noted that reaching these conversion rates at lower temperatures is an indication of better catalyst performance. He says, “We examined multiple oxidation temperatures from 700 C to 1,100 C. Rhodium/alumina catalyst activity declined significantly with 50% conversion rates declining by 60 C or more for all exhaust gases at a temperature of 950 C.”
The researchers used atomic-resolution scanning transmission electron microscopy, electron energy loss spectroscopy and extended X-ray absorption fine structure spectroscopy to identify the formation of rhodium aluminate, which is the cause of catalyst deactivation. In the presence of hydrogen at temperatures ranging from 600 C to 800 C, the rhodium/alumina catalyst can be regenerated through a reduction process.
Li says, “There are two pathways for reduction of rhodium activity. Rhodium nanoparticles sinter into larger particles reducing their catalytic surface area. The second pathway is for rhodium nanoparticles to decompose into rhodium atoms that migrate into the alumina sublattice to form rhodium aluminate, which is hard to reduce.”
He adds, “The extent of catalytic deactivation is dependent on the concentration of rhodium present. Under low-load conditions (e.g., 0.6 weight % rhodium), the catalyst will be nearly completely deactivated, which involves the complete consumption of rhodium aluminate. At a higher loading of 5%, partial catalytic activity was observed because the primary decomposition pathway to rhodium aluminate saturated all accessible vacancy sites on the alumina leaving the remaining rhodium nanoparticles to sinter.”
Figure 1 shows a schematic of the deactivation and regeneration of rhodium/alumina.
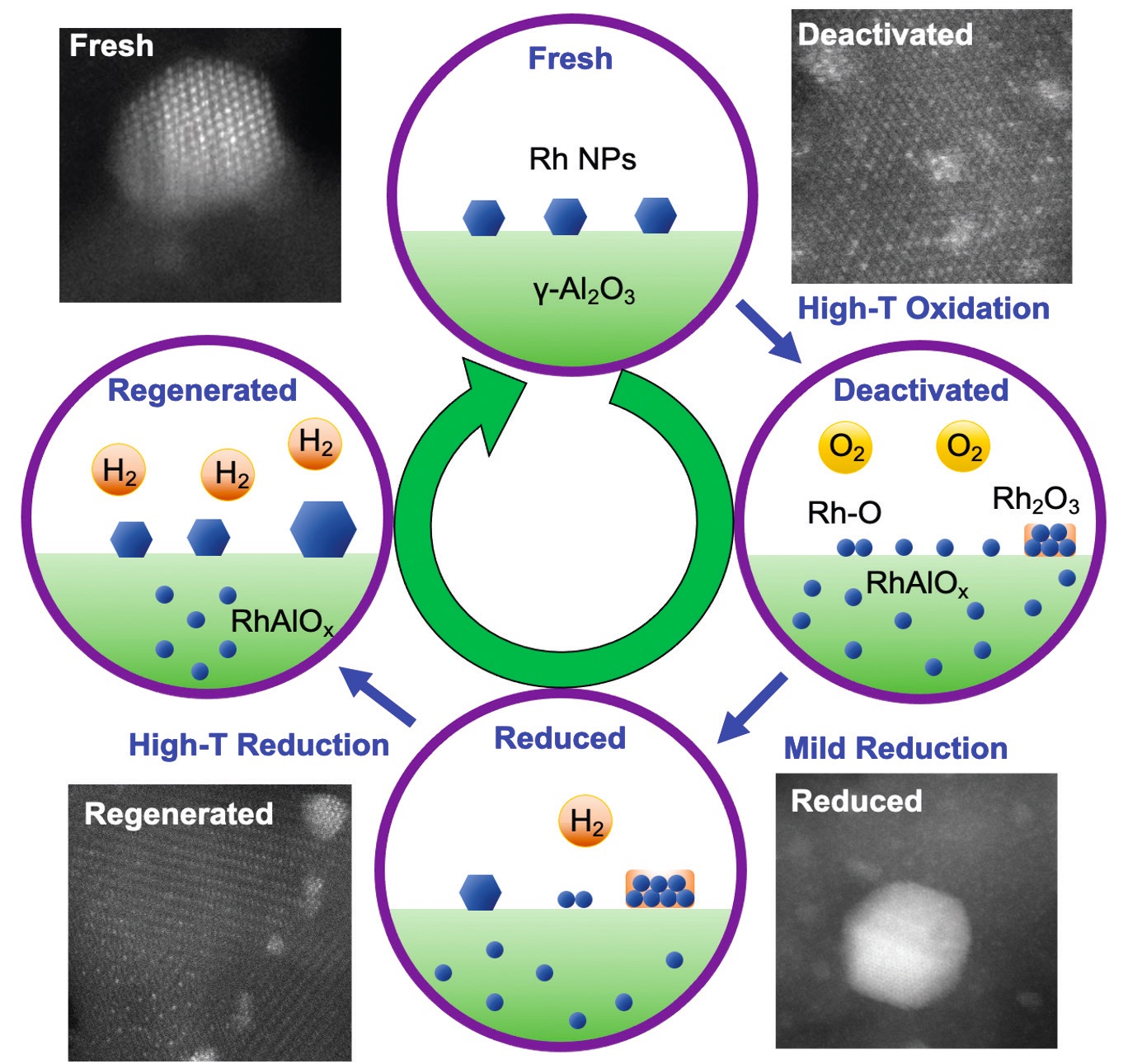 Figure 1. A schematic of the deactivation and regeneration of rhodium (Rh)/alumina three-way catalysts is shown. Figure courtesy of Cheng-Han Li and taken from his Ph.D. dissertation, “Advanced Characterization of the Deactivation and Regeneration Mechanism in Rh-Based Three-Way Catalyst” – The Ohio State University (2022).
Figure 1. A schematic of the deactivation and regeneration of rhodium (Rh)/alumina three-way catalysts is shown. Figure courtesy of Cheng-Han Li and taken from his Ph.D. dissertation, “Advanced Characterization of the Deactivation and Regeneration Mechanism in Rh-Based Three-Way Catalyst” – The Ohio State University (2022).
Future work may concern an
in situ study using transmission electron microscopy to further clarify how rhodium atoms migrate into alumina to form rhodium aluminate. Li adds, “We have been working on another project that focuses on how the effects of phosphorus, potentially from oil additives, on rhodium/alumina may influence catalytic activity.”
Li hopes that this work will facilitate the design of new catalysts that specifically focuses on cation additives to saturate the octahedral vacancy sites of alumina and, hence, the prevention of rhodium aluminate formation.
Additional information can be found in a recent article
2 or by contacting Jinschek at
jojin@dtu.dk.
REFERENCES
1.
Canter, N. (2020), “Determination of active vehicle exhaust catalytic sites,” TLT,
76 (11), pp. 14-15. Available
here.
2.
Li, C., Wu, J., Getsoian, A., Cavataio, G. and Jinschek, J. (2022), “Direct observation of rhodium aluminate (RHAlO
x) and its role in deactivation and regeneration of Rh/Al
2O
3 under three-way catalyst conditions,”
Chemistry of Materials, 34 (5), pp. 2123-2132.