KEY CONCEPTS
•
Water is a very effective heat transfer fluid when boiling, but this phase change cannot be extended to high temperature applications.
•
The Leidenfrost effect, when a liquid such as water levitates above its own vapor in a two-phase system, can extend the boiling to 150 C.
•
Introduction of ice to create a three-phase Leidenfrost effect (with water and vapor) has now been found to extend boiling to 550 C, which should represent a dramatic increase in heat transfer efficiency.
The growth of electronics in many applications (such as electric vehicles) is leading to growing concerns about how to dissipate the high levels of heat generated during use. Electric motors and batteries operate optimally at ambient temperatures, which makes it essential to dissipate heat generated as efficiently as possible.
This trend has placed more attention on the use of heat transfer fluids. A previous TLT article
1 discussed the basic functions of heat transfer fluids and their specific use in electric vehicles. Heat transfer fluids move heat from one location to another for the purpose of either lowering or raising the temperature in a specific application.
Water is one of the most effective heat transfer fluids because it can absorb a lot of heat when boiling. But the boiling of water cannot be extended to high-temperature applications.
One of the more interesting properties of water when heated is known as the Leidenfrost effect. Jonathan Boreyko, assistant professor of mechanical engineering at Virginia Tech in Blacksburg, Va., says, “The Leidenfrost effect was first discovered by Johann Leidenfrost, a physician and theologian in the 18th Century. Leidenfrost found that a liquid such as water, when placed near a heated material, levitates above its own vapor. This effect also can be seen with substances that sublime such as carbon dioxide where dry ice also can levitate on its own vapor.”
In experiments evaluating droplets of water placed on a heated aluminum plate, Boreyko and his colleagues observed that the Leidenfrost effect can take place at temperatures up to 150 C, which is significantly above the boiling point of water. This phenomenon occurs because the water droplet is not in direct contact with the hot metal surface due to the cushion established by the vapor.
Boreyko says, “In effect, the Leidenfrost effect involves the transition through a series of different types of boiling. Initially, nucleate boiling (conventional boiling) is occurring as the temperature starts to rise. But then a transition starts to occur as nucleate boiling is partially replaced by film boiling. The temperature where film boiling completely replaces nucleate boiling is known as the Leidenfrost point. Past this temperature, the liquid is fully levitated and completely loses any ability to undergo nucleate boiling.”
The most efficient heat transfer occurs during nucleate boiling as vapor is able to direct heat away from a specific source such as the aluminum plate. The challenge to improve heat transfer is to increase the Leidenfrost point, which is 150 C for a two-phase system (water and vapor) in this experimental setup.
Three phases
The researchers have been conducting work to determine how to facilitate ice removal
2 and decided that it would be interesting to find out if using ice provides any impact on the Leidenfrost effect. Boreyko says, “We combined our research on ice with the Leidenfrost effect to see if ice can be levitated in a similar manner to a water droplet.”
If ice can levitate, then the researchers will be able to generate a three-phase Leidenfrost effect where ice levitates on its meltwater, which, in turn, levitates on its evaporative vapor. An experiment was set up where frozen ice disks with a radius of either 8 millimeters (mm) or 25 mm and a thickness of either 7 mm or 12 mm were prepared in polycarbonate petri dishes placed in a freezer.
The ice disks were gently placed on a heated aluminum stage through the use of a toothpick that was suspended in the water prior to freezing. A ring was used to prevent the ice disks from gliding off the metal surface.
The researchers found that boiling was suppressed up to a temperature of 150 C. Nucleate boiling was observed between 150-450 C followed by a transition boiling range between 450-550 C. The Leidenfrost point is 550 C, which represents a dramatic increase of 400 C compared to the two-phase Leidenfrost effect with water and vapor.
Boreyko says, “After spending three years modeling the three-phase Leidenfrost effect, we determined the reason for the significant increase in the Leidenfrost point when ice is included with water and vapor. At the boundary between melt water and vapor, the temperature is 100 C, the boiling point of water at room pressure. With ice now on top of the water, the temperature at the boundary between ice and water is 0 C, the freezing point of water. A microscopic water film must be present between the ice and the vapor, with a temperature of 100 C on the bottom and a temperature of 0 C on top. The reason for the extended Leidenfrost point is that most of the heat added to the three-phase Leidenfrost effect is needed to maintain this water film at the extreme temperature range. Vapor formation is minimized leading to an increase in the boiling range.”
The significance of this result is that the inclusion of ice extends the period of nucleate boiling, which should improve heat transfer efficiency. Mojtaba Edalatpour, graduate Fellow at Virginia Tech, indicates this finding means that ice can be used in a number of applications to improve the efficiency of heat transfer. He says, “We have seen that a nuclear facility is utilizing a block of ice imbedded on the reactor to facilitate cooling if needed to dissipate heat and in case of an emergency.”
A second application is to use ice in improving the quenching (heat treatment) of metals. This may improve the ability to control the quenching process maximizing strength while minimizing brittleness.
Edalatpour indicates that ice can be applied as a spray of particles or as an aerosol of ice chips. He says, “The experiments we have conducted assess the static Leidenfrost effect. We are now releasing ice spheres about the aluminum plate at different distances and velocities to determine what this will do to the Leidenfrost point
(see Figure 2). This change in physics represents an evaluation of a dynamic Leidenfrost effect.”
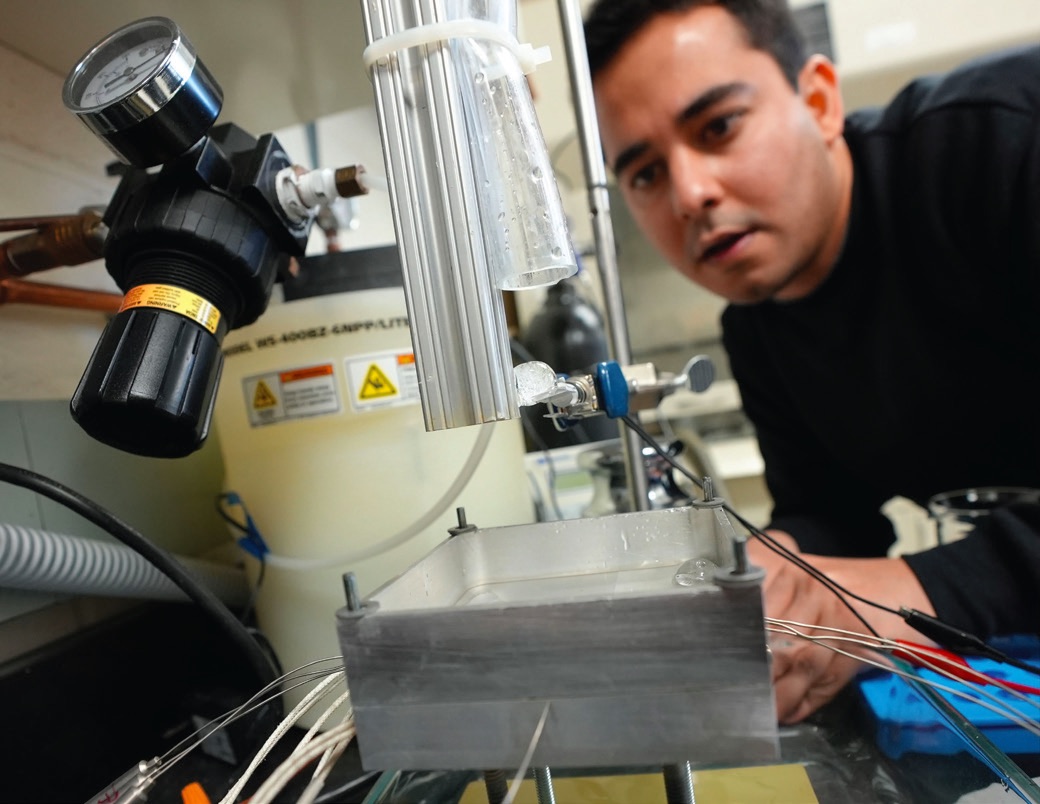 Figure 2. The three-phase Leidenfrost effect has demonstrated that the heat transfer capability of water can be extended. New experiments are underway to evaluate the dynamic Liedenfrost effect through dropping ice spheres at different distances and velocities onto an aluminum plate. Figure courtesy of Virginia Tech.
Figure 2. The three-phase Leidenfrost effect has demonstrated that the heat transfer capability of water can be extended. New experiments are underway to evaluate the dynamic Liedenfrost effect through dropping ice spheres at different distances and velocities onto an aluminum plate. Figure courtesy of Virginia Tech.
Additional information can be obtained from a recent article
3 or by contacting Boreyko at
boreyko@vt.edu.
REFERENCES
1.
Canter, N. (2021), “Heat transfer fluids: Growing in visibility and importance,” TLT,
77 (12), pp. 28-40. Available
here.
2.
Canter, N. (2021), “Ice removal through suspension in air,” TLT,
77 (11), pp. 16-17. Available
here.
3.
Edalatpour, M., Cusumano, D., Nath, S. and Boreyko, J. (2022), “Three-phase Leidenfrost effect,”
Physical Review Fluids, 7 (1), 014004.