KEY CONCEPTS
•
A new approach, known as the wet-electrostatic (WE) method, was developed to eliminate the formation of inverted cracks in nickel nanolattices that have the strength of titanium but are light enough to have a density similar to water.
•
Glycerol was added to the colloidal solution used to ensure proper formation of the template.
•
To overcome repulsion of the deposited nickel metal filling into opals, positively charged particles containing amidine groups replaced negatively charged particles in the colloidal solution.
The growing use of electronics and electronic devices is leading to the need for the development of lighter materials that can be used in their construction. These lighter materials also need to be stronger than what is currently used. James Pikul, assistant professor in the department of mechanical engineering and applied mechanics at the University of Pennsylvania in Philadelphia, Pa., says, “The appeal of these high surface area materials is that they can potentially be used in applications such as electric vehicles to not just reduce the automobile’s weight but also enhance cooling through more efficient heat transfer without sacrificing strength.”
A prime example, discussed in a previous TLT article,
1 was the preparation of a nickel-based material that has a porous structure similar to wood. Pikul led a research group that produced such a material, which was designated as metallic wood. He says, “This material has a strength comparable to titanium but is light enough to exhibit a density similar to water. The strength originates in the placement of nanoscale nickel struts within regularly spaced cell-sized pores that reduce the material’s density. The strength of these struts increases as they are reduced in size to the nanoscale.”
The challenge in working with nanolattices such as metallic wood is to make larger quantities. Pikul says, “The current process for manufacturing nanolattices is to use additive manufacturing at the nanoscale. The use of 3D printing provides precise control but is time consuming, which makes it impractical to make square meters of material.”
A self-assembly technique developed by Pikul and his colleagues that fills the voids of self-assembled colloidal templates (prepared from polystyrene) with metallic lattices provides small quantities but is vulnerable to the formation of dense cracks that can produce defects known as inverse crack structures. He says, “In the fabrication of lattice templates, a colloidal solution of negatively charged nanoparticles is self-assembled on a negatively charged surface. As the water evaporates, dried opals form from the close packing of the nanoparticles, but large cracks in these structures form as the waterline recedes. Previously, we could not avoid these cracks, but we could still make small areas of metallic wood (domains less than 0.01 millimeter) by sintering the particles, electrodepositing nickel into the voids and then removing the template.”
Pikul points out that a similar effect is seen as water evaporates from sand causing cracks to form.
A new approach for eliminating the formation of these defects has now been developed that does not sacrifice the high strength and low density of the nanolattices.
Wet-electrostatic method
Pikul and his colleagues developed a new approach known as the wet-electrostatic (WE) method that eliminates the formation of inverted cracks. Initially, the researchers added a low-vapor-pressure alcohol such a glycerol and ethylene glycol to the colloidal solution used to form the template. Pikul says, “These alcohols, which contain multiple hydroxyl groups, keep the template wet while not interfering with the self-assembly process.”
Establishing the optimum alcohol concentration is very important to ensure the template forms properly. Pikul says, “Too low of an alcohol concentration will lead to inverted crack formation while too high of a concentration will not allow for self-assembly.” The researchers found that 0.06% by volume of glycerol is ideal.
The elimination of inverted cracks did not completely solve the issue of producing nickel nanolattices. During the electrodeposition process, a negative potential is applied to the substrate to facilitate the reduction of nickel ions into nickel metal. Negatively charged particles based on sulfated polystyrene, sulfated polymethyl methacrylate and silica are commonly used to make opals. Unfortunately, the applied negative potential prevents the deposited metal from filling into the opals because the negatively charged template pushes away from the surface.
Pikul says, “We prepared positively charged particles containing amidine groups to replace the negative charged particles in the colloidal solution. This eliminated the delamination problem and allowed nickel to nanolattices to be formed without inverted cracks.”
The WE method enabled the researchers to prepare much larger nickel nanolattices with a crack-free area 20,000 times bigger than previously reported using the currently accepted method. The top images in Figure 2 show the honeycomb structure of the nickel nanolattices. Pikul says, “Resolving the inverted crack problem has given us the opportunity to manufacture larger structures and to conduct a macroscopic study of the nanolattice tensile mechanical properties. The bottom left image in Figure 2 shows a dog bone structure used in tensile testing. The spectrum of color shown on the dog bone is an indication of the uniform nature of the nickel nanolattice that displays well-aligned polycrystalline structures separated by small-angle boundaries.”
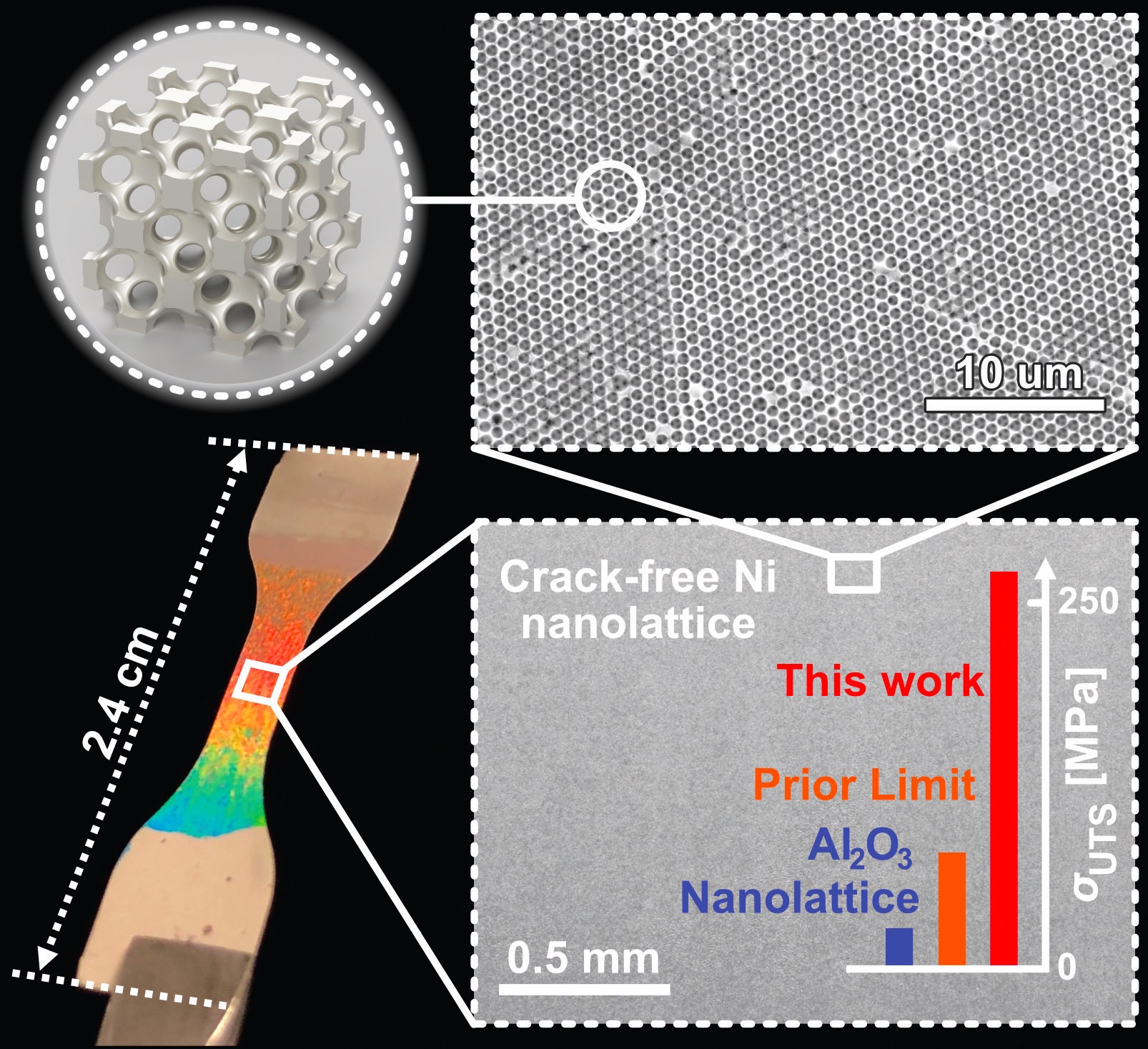 Figure 2. Tensile testing using a dog bone (see bottom left image) shows that a nickel lattice prepared by the wet electrostatic method (see top two images) generates superior tensile mechanical properties (see the graph on the bottom right) compared to a nickel lattice produced by the existing method and a nanolattice based on alumina. Figure courtesy of the University of Pennsylvania.
Figure 2. Tensile testing using a dog bone (see bottom left image) shows that a nickel lattice prepared by the wet electrostatic method (see top two images) generates superior tensile mechanical properties (see the graph on the bottom right) compared to a nickel lattice produced by the existing method and a nanolattice based on alumina. Figure courtesy of the University of Pennsylvania.
A tensile strength of 260 megapascals was achieved with the nickel nanolattice, which approaches the theoretical limit for porous nickel. The bottom right image in Figure 2 illustrates the dramatic improvement in tensile strength compared to a nickel nanolattice produced by the existing method and for a nanolattice based on alumina.
Future work will concern identifying real-world applications for crack-free metallic wood. Pikul says, “Our approach can be used not just on nickel but on other metals including copper and gold. We also are working to scale up the process for manufacturing nanolattices beyond what was accomplished in this study, and applying these materials to make better sensors and mechanical devices.
Additional information on this work can be found in a recent article
2 or by contacting Pikul at
pikul@seas.upenn.edu.
REFERENCES
1.
Canter, N. (2019), “New material with strength of titanium and density of water,” TLT,
75 (5), pp. 16-17. 17. Available
here.
2.
Jiang, Z. and Pikul, J. (2021), “Centimetric-scale crack-free self-assembly for ultrahigh tensile strength metallic nanolattices,”
Nature Materials, 20 (11), pp. 1512-1518.