Meet the Presenter
This article is based on a webinar presented by Auburn University on June 1, 2021. The webinar series, titled The Beard Tribology Webinar Series, is named after the late Ralph Beard, who pioneered the study of tribology at Auburn. 2D MXenes - Tunable Mechanical and Tribological Properties is available at www.eng.auburn.edu/programs/tribology/beard-tribology-webinar.html.
Andreas Rosenkranz is a professor for material-oriented tribology at the University of Chile. He obtained his diploma in 2010 and a doctorate in 2015 in materials science and engineering from the Saarland University in Germany. His main research focuses on the possibility of reducing friction and wear by either surface functionalization or the use of nanoparticles. He has authored more than 120 peer-reviewed journal publications. He is an Alexander von Humboldt Research Fellow and serves as an academic editor for Frontiers of Chemistry, Applied Nanoscience and Industrial Lubrication and Tribology. In 2015, he moved first to Santiago, Chile, to work on the tribology of hard coatings at the Catholic University Santiago de Chile. Then, he went to the University of California to work on the tribological performance of magnetic discs, especially on very thin carbon coatings (Feodor Lynen Research Fellowship from the Alexander von Humboldt Foundation). In 2018, he rejoined the University of Chile to establish the field of tribology in Chile.
You can reach Rosenkranz at arosenkranz@ing.uchile.cl.

Andreas Rosenkranz
KEY CONCEPTS
•
MXenes get their name from a bulk crystal called MAX phase and their similarity to graphene.
•
MXenes have good conductivity and an outstanding ability to store an electrical charge.
•
The number of possible, stable MXene formulations runs into the hundreds of thousands.
There is a new class of 2D materials, called MXenes, and pronounced max-eens. They get their name from a bulk crystal called MAX phase and their similarity to graphene. Prior to their first synthesis in 2011, there was no evidence that these 2D-layered materials even existed.
MXenes, which are ceramics, comprise one of the most expansive classifications of 2D materials.
They can be fabricated on a micron scale and have interesting mechanical properties that are halfway between graphene and graphene oxides.
While the unique properties of MoS
2, graphene and other 2D structures have been recognized for a long time, MXenes represent a leading-edge class of 2D materials with a wide range of properties that exceed their 2D predecessors. This creates a path for a better grasp of the property differences between 2D and 3D substances, possibly culminating in the discovery of useful properties and applications for 2D nitrides, carbides and related materials.
MXenes originate from MAX phase.
1 While MAX phases have been widely researched as 3D materials, the breakthrough came when researchers first removed the aluminum from titanium-aluminum carbides. The result of this “exfoliation” procedure was to spread out the layered carbide material into 2D Ti
3C
2 nanosheets.
MXenes are very durable, one of the strongest materials in their class. Unlike graphene, MoS
2 and other 2D ceramics, MXenes have good conductivity and an outstanding ability to store an electrical charge. This is due to the fact that they are formed into molecular sheets composed of nitrides and carbides of titanium or other early transition metals. MXenes also excel at blocking electromagnetic interference, resisting bacteria and purifying water.
To use 2D materials that are either particles, nanosheets or nanoflakes, the question becomes how to deposit these nanomaterials on a surface. One method is mechanical exfoliation. There also are coating techniques such as spin coating and spray coating and also more sophisticated methods such as atomic layering and physical vapor deposition. Current research and development is focused on coatings.
This article is based on a webinar, titled 2D MXenes - Tunable Mechanical and Tribological Properties, and presented by Andreas Rosenkranz—professor for material-oriented tribology at the University of Chile—and Auburn University. See Meet the Presenter for more information.
The origin of MXenes
MXenes have a very defined and precise tachometry. In order to obtain this MAX phase, one needs to use aluminum, carbon and titanium powder. They are put in an oven and heated to high temperatures, i.e., 1,600 C for two hours. This creates a beautiful layer-like structure with the blue atoms representing the titanium atoms, the black atoms representing the carbons and the red atoms representing the aluminum layers. So, there is a titanium layer followed by a carbon layer followed by an aluminum layer.
The interesting thing is the bonding characteristics in the formed Ti
3A
lC
2. There is bonding between titanium and carbon and bonding between titanium and aluminum. So, in this sense, regarding the bonding strength and bonding characteristics between titanium and carbon, there is a mixed bonding, meaning there are contributions of metallic, ionic and covalent bonds while the titanium-aluminum bond is mainly metallic. This creates minor differences in their respective bonding strength with the titanium-carbon a little stronger bond than the titanium.
MXene synthesis
Researchers came up with the idea of selectively removing just the aluminum layers and focusing only on the titanium and carbon in the system. This is how MXene synthesis works: only the aluminum layers are removed through selective etching. One example is to put the MAX powder in a highly concentrated hydrofluoridic acid and wait for 24 hours. The synthesis and processing steps can be summarized as follows:
1.
Synthesis of the MAX phase
2.
Selective etching to obtain the multi-layered MXenes
3.
Exfoliation/delamination—to obtain few and single-layer MXenes
4.
Final processing.
There is a broad range of stoichiometries that can interchange early transition metals. The carbon to nitrogen ratio can be experimented with and, in the future, it may be possible to experiment with the surface to manipulate the resulting surface terminations.
While there are millions of possible arrangements for MXene transition metals, the challenge is to identify versions that are highly stable with the lowest formation energy. Even so, researchers estimate that there are hundreds of thousands of stable MXene compounds awaiting development.
Applications
Most MXene applications relate to energy storage (fabrication of batteries or supercaps). Other general applications include:
•
catalysis,
•
electromagnetics,
•
electronics,
•
mechanical/tribological applications,
•
environmental,
•
sensors and
•
biology.
MXenes’ tunable mechanical properties, such as mechanical stiffness, monolayer thickness, bond stiffness and elastic modulus, are very important for most applications. However, there has been very little research on these properties.
Because of their unique properties, MXenes have a variety of specific identified applications, and the list is only growing longer.
•
Wastewater treatment/desalination: Ti3C2 can tap the energy of sunlight to purify water through evaporation in a highly energy efficient manner.
•
Battery technology and energy storage: MXenes have a very high lithium-ion capacity in addition to an extremely high-charging rate cyclability that outperforms graphite.
•
MXene electrodes: Electrode configurations employing MXenes allow batteries to charge much more quickly.
•
Triboelectric nanogenerators: MXenes could repurpose wasted frictional energy because they have high electrical conductivity, and they can uptake electrons during contact with materials such as polymers.
•
Conductive coatings: MXenes can be used to create a conductive coating that will maintain performance under heavy bending and stretching.
•
Gas sensors: MXenes are perhaps the most sensitive gas sensors, with the ability to detect very low concentrations.
MXenes as solid lubricants
Tribological properties of MXenes were first studied around 2015. Thus, research into the application of MXenes in tribology is in its infancy. Most important to tribology is that MXenes can achieve near superlubricity. While MXenes can take the form of a lubricant additive, solid lubricant or composite, to date, the success of MXenes as a lubricant additive has been limited. Solid lubricants may be the best success story so far in the application of MXenes to tribology, although composites also have potential.
Two-dimensional MXenes are easily shearable, which does make them a good choice for solid lubricants. There is a significant improvement over liquid lubricants related to service life and energy efficiency through the usage of simple multi-layer MXene solid lubricant coatings
(see Figure 1).
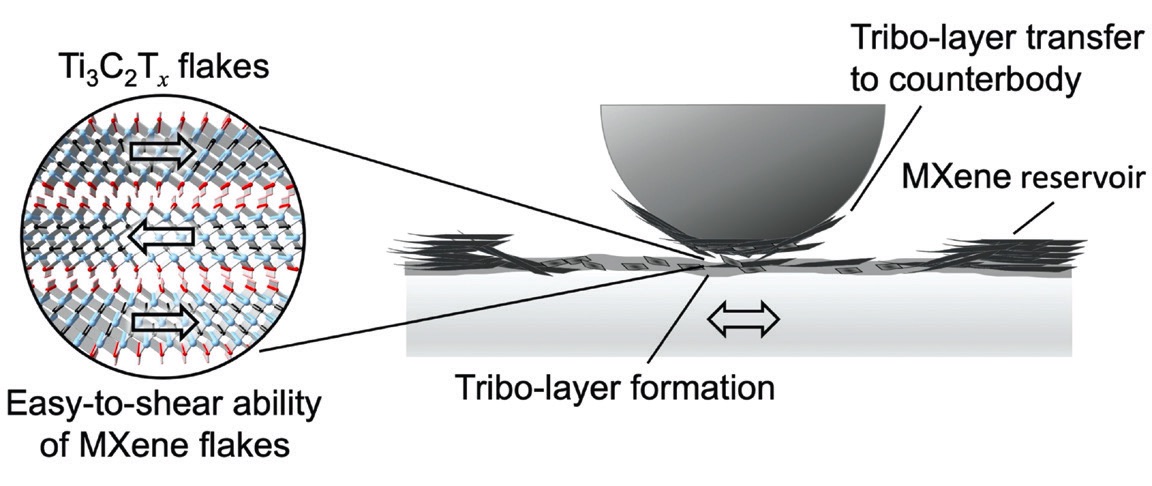 Figure 1. The mechanism through which multi-layered MXenes form a solid lubricant coating. Figure reprinted with permission from Grützmacher, P. G., Suarez, S., Tolosa, A., et al. (2021), “Superior wear-resistance of Ti3C2Tx multilayer coatings,” ACS Nano, 15 (5), pp. 8216-8224. Copyright 2022 American Chemical Society.
Figure 1. The mechanism through which multi-layered MXenes form a solid lubricant coating. Figure reprinted with permission from Grützmacher, P. G., Suarez, S., Tolosa, A., et al. (2021), “Superior wear-resistance of Ti3C2Tx multilayer coatings,” ACS Nano, 15 (5), pp. 8216-8224. Copyright 2022 American Chemical Society.
Limitations related to liquid lubricants include:
•
environmental issues (disposal),
•
necessary confinement, limited temperature range,
•
limited pressure range and
•
degradation in service.
Because of this, many industries tend to move to solid lubricants. While graphene is a proven solid lubricant, studies in industrial applications show that MXenes perform better than ultra-low friction materials. MoS
2 and diamond-like carbons (DLCs) have been used for decades—however, once they start to wear off, the protection is reduced. This is not an issue with MXenes
(see Figure 2).
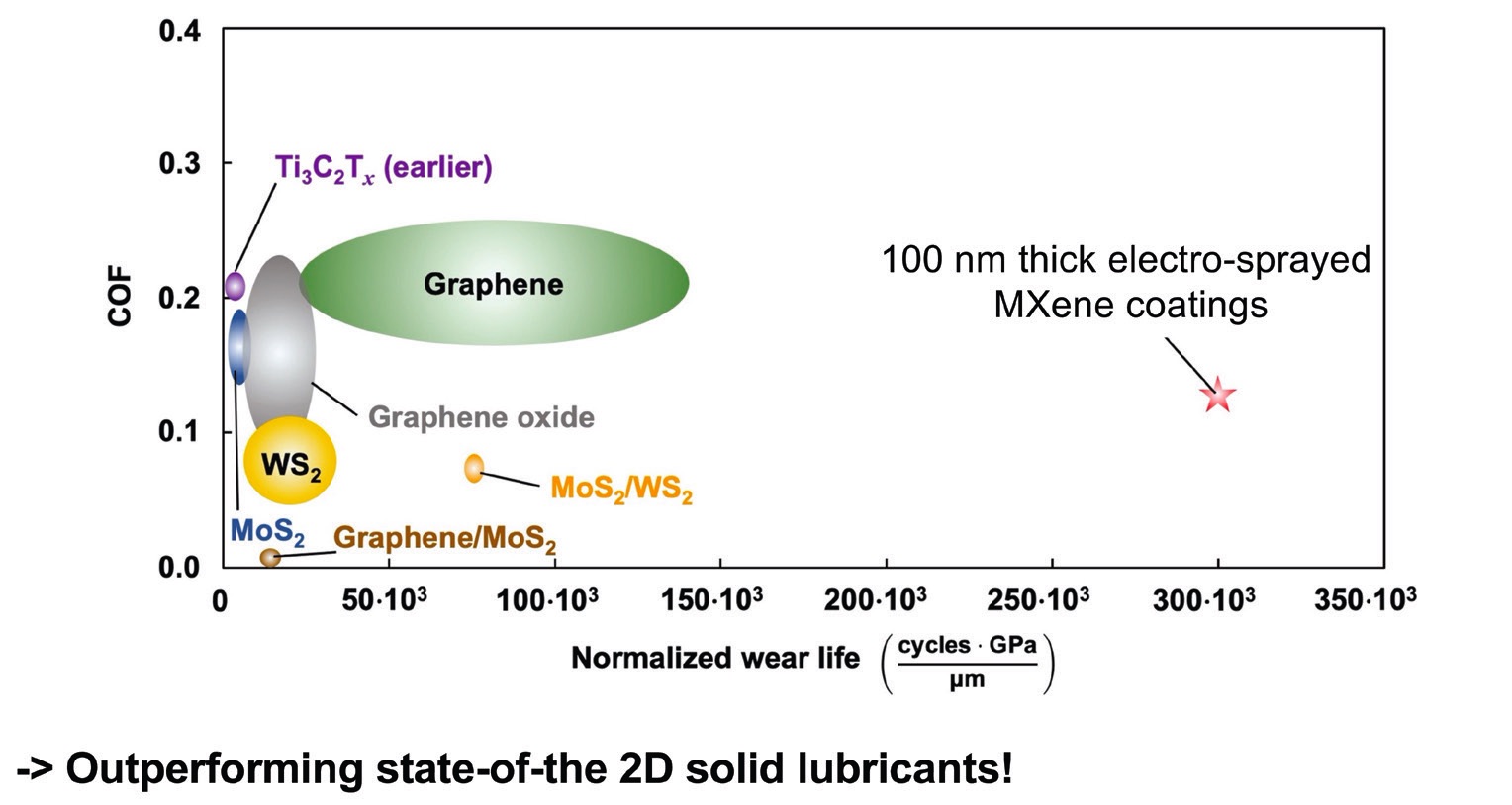
Figure 2. The benchmark for multi-layered MXenes to form a solid lubricant coating. Figure reprinted with permission from Grützmacher, P. G., Suarez, S., Tolosa, A., et al. (2021), “Superior wear-resistance of Ti3C2x multilayer coatings,” ACS Nano, 15 (5), pp. 8216-8224. Copyright 2022 American Chemical Society.
MXenes make an excellent solid lubricant that is extremely durable and performs under the most difficult conditions. For example, while lubricating oil would evaporate immediately during space missions, MXene as a fine powder would be both effective and stable. Another decisive advantage is heat resistance—this holds true in severe conditions, such as steel mills.
The future for MXenes looks bright. Their energy storage and purification applications, in particular, align well with the current focus on electric vehicles and sustainability. Researchers believe they will even enable a new generation of flexible and printable electronic and opto-electronic devices.
REFERENCE
1.
MAX phases, which are polycrystalline nanolaminates of ternary nitrides and carbides, get their name from their general formula M
n+1AX
n.
Jeanna Van Rensselar heads her own communication/public relations firm, Smart PR Communications, in Naperville, Ill. You can reach her at jeanna@smartprcommunications.com.