KEY CONCEPTS
•
Electric field stimulation was used to deactivate wet adhesives by controlling the flow of ions in an aqueous medium.
•
Upon application of an electric field, the strength of a wet adhesive declined by nearly 100%.
•
The catechol-based adhesive is oxidized during the process, leading to a change in color from white to red.
Adhesion is an important property for lubricants that need to operate at the interface between a surface and the fluid. The ability of the lubricant to adhere to the surface of a machine is a critical factor in minimizing friction and wear.
Displaying good adhesion in aqueous environments can be more challenging because water is an inferior lubricant. Another challenge is to develop reversible or smart adhesives that will operate in a particular application for a finite time frame and then, through some process, be released so that they can be used in a different application at a later time.
In a previous TLT article,
1 a switchable electronically controlled capillary adhesion device was developed. The researchers emulated the behavior of a leaf beetle by combining the surface tension force established by the insect with electronic control to enable the adhesion to be reversible. An electroosmotic pump was utilized to force water droplets through an orifice, which formed liquid bridgelets that make contact with a surface.
Bruce Lee, associate professor of biomedical engineering at Michigan Technological University in Houghton, Mich., says, “A smart adhesive displays reversible properties through interaction with an external stimulus such as light irradiation or temperature.”
Most currently available smart adhesives operate effectively on dry surfaces but are limited when water is present. Lee says, “The problem with finding effective wet adhesives is that water competes very effectively with the surface of a substrate. The result is that adhesives will prematurely fail as most of us have noted when a Band-Aid on a finger will come off during washing.”
In evaluating potential wet adhesive candidates, Lee has turned to Mother Nature. He says, “Catechol derivatives have emerged because many natural proteins from organisms such as marine mussels and sandcastle worms secrete protein-based adhesives containing a unique amino acid (3,4-dihydroxyphenyalanine) that contains this functionality. Our interest is in the preparation of synthetic derivatives that mimic these natural proteins.”
In initial experiments, Lee found that changing the pH of an aqueous medium will directly affect the adhesive properties of catechol derivatives. He says, “The reduced form of catechol exhibits good wet adhesion when the pH is acidic. But, as the pH increases, catechol is oxidized, reducing its interfacial binding strength to an inorganic surface to such an extent that it loses adhesion. At a pH range between 5 and 6, there is an 80% reduction in wet adhesion. This only increases as the pH rises above neutral into the alkaline range.”
But pH is not an effective way to control adhesion because of the slow diffusion of hydrogen and hydroxyl ions. A new approach has now been devised to deactivate adhesion more effectively in the presence of water.
JKR contact mechanics test
Lee and his colleagues have determined that catechol-based wet adhesives can be deactivated through the use of electric field stimulation. Lee says, “Electricity controls the flow of ions in an aqueous medium. This enable us to control the pH and, as a result, control the oxidation of catechol.”
The researchers used a device known as the Johnson-Kendall-Roberts (JKR) contact mechanics test to evaluate the use of an electrical field to deactivate the adhesive. Lee says, “JKR is an established test that is used to evaluate the interfacial binding properties of an adhesive. This test is focused on the interface between the adhesive and the substrate. The JKR test is very suitable for examining wet adhesives because it is a more controlled system.”
In the test setup, the researchers prepared a model adhesive based on the monomer, dopamine methacrylamide, which contains the catechol functionality. This monomer is copolymerized with an acrylate monomer and then crosslinked with an acrylamide derivative to produce the adhesive. A titanium electrode is used as a cathode and is brought into contact with the adhesive wetted with a 0.1 molar sodium chloride solution buffered at a pH of 7.5. A platinum wire is used as the anode.
Lee says, “We used titanium as the cathode because it is well established that this metal binds readily to catechol derivatives.” The experimental setup is shown in Figure 3.
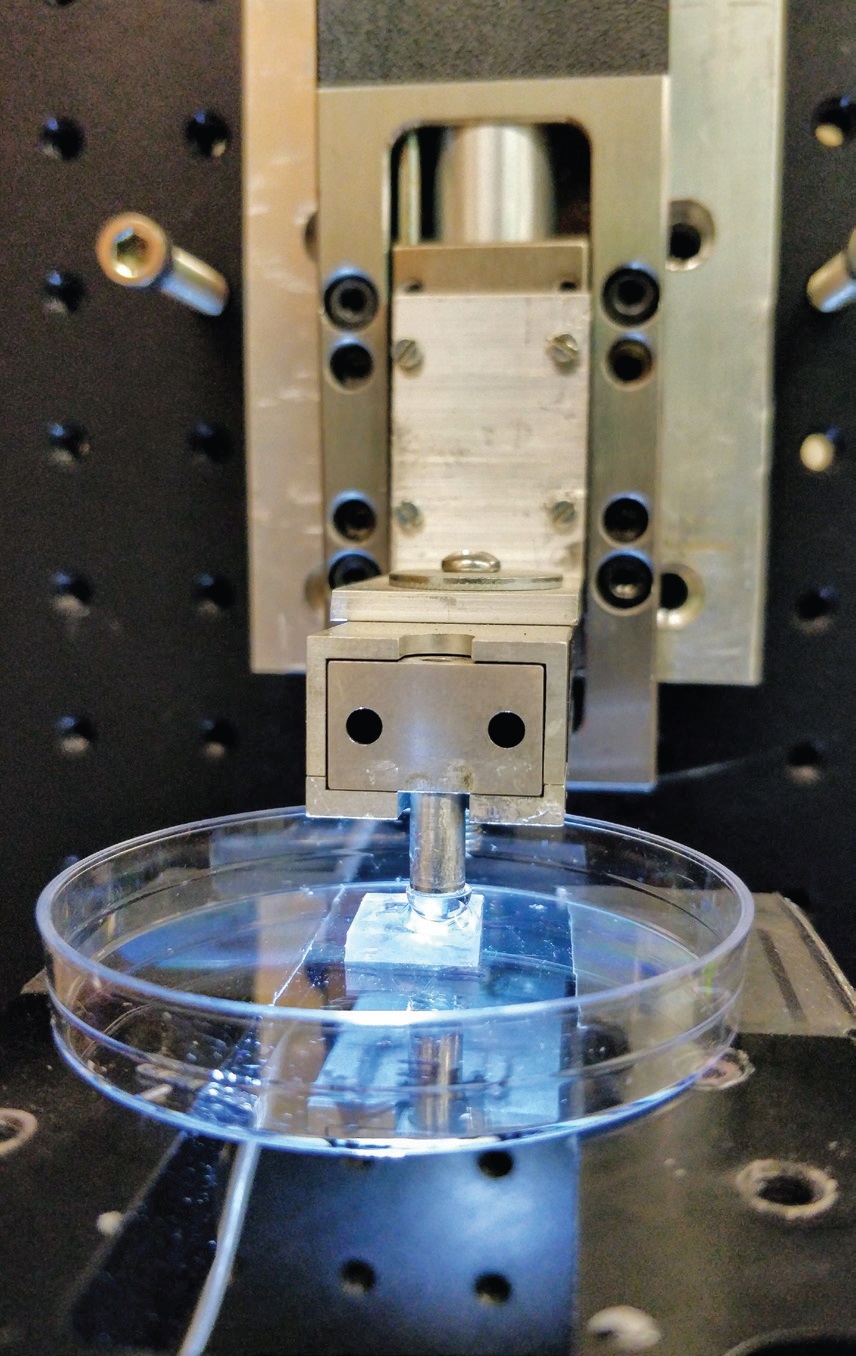 Figure 3. The experimental setup using the JKR contact mechanics test to evaluate wet adhesives is shown. Figure courtesy of Michigan Technological University.
Figure 3. The experimental setup using the JKR contact mechanics test to evaluate wet adhesives is shown. Figure courtesy of Michigan Technological University.
Prior to application of the electrical field, the adhesive exhibited strong interfacial binding with a maximum tensile load of 70 millinewtons. Upon application of up to nine volts, the strength of the adhesion declined by nearly 100%.
The researchers noted that the color of the catechol, in close proximity to the cathode after oxidation, changed from white to red. Lee says, “This phenomenon is an indication of how the JRK test is focused on evaluation right at the interface. The surface of the adhesive near the anode remained white.”
In an attempt to reduce the catechol derivative so that it can act as a reversible adhesive, the researchers reversed the process. Lee says, “We reversed the process by making the titanium electrode the anode. Unfortunately, two competing processes did not permit the resumption of adhesion. The pH of the aqueous medium near the titanium anode did acidify, which could lead to enhanced adhesion, but the catechol in the adhesive was oxidized at the same time reducing adhesion.”
The researchers are now working to reactivate the adhesive. Lee says, “Our objective is to figure out how to turn a wet adhesive on and off. A second goal is to enable the adhesive to be effective at an alkaline pH.”
Additional information can be found in a recent article
2 or by contacting Lee at
bple@mtu.edu.
REFERENCES
1.
Canter, N. (2010), “Switchable adhesion: Ability to walk on walls,” TLT,
66 (95), pp. 14-15.
2.
Bhuiyan, M., Roland, J., Liu, B., Reaume, M., Zhang, Z., Kelley, J. and Lee, B. (2020), “In Situ Deactivation of Catechol-Containing Adhesive Using Electrochemistry,”
Journal of the American Chemical Society,
142 (10), pp. 4631-4638.